University of Southampton Research Repository
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

The River Conwy Catchment Management Plan Action Plan; 1995
U > ^ [ J 2 S THE RIVER CONWY CATCHMENT MANAGEMENT PLAN ACTION PLAN; 1995 NRA I National Rivers Authorii Welsh Region CONTACTING THE NRA The national head office of the NRA is in Bristol Enquiries about the Conwy Catchment Management Plan should be directed to: Telephone: 01454 - 624400 Dr Ken Jones, The Welsh Region head office is in Cardiff Area Planning and Support Services Coordinator, Telephone: 01222 - 770088 H ighfield, Priestley Road, The Area Manager for the Northern Area of the Welsh Caernarfon, Region is: G w ynedd, Roger Thomas, LL55 1HR Bryn Menai, Holyhead Road, B an g o r, G w y n edd . L L 57 2EF NRA Copyright Waiver. This report is intended to be used widely and may be quoted, copied or reproduced in any way, provided that the extracts are not quoted out of context and due acknowledgement is given to the National Rivers Authority. Acknowledgement:- Maps are based on the 1992 Ordnance Survey 1:50,000 scale map with the permission of the Controller of Her Majesty’s stationary Office © Copyright. WE 2k E AMOM 3 95 Ill THE N R A' S VISION FOR THE CONWY CATCHMENT The Afon Conwy catchment features immediate flow will be safeguarded through the careful attractions for those whose interests lie outdoors, consideration of all abstraction licence applications ranging from the simple enjoyment of the often which predominantly have related to hydro-electric breathtaking scenery through to active participation in power schemes in recent times, and property will be sports such as fishing, canoeing and climbing. protected from flooding to a standard which is consistent with the land use and economically The catchment is also home to a population of about justifiable. -

Appendix I: European Site Characterisations
Appendix I HRA Anglesey & Gwynedd Joint LDP Appendix I: European Site Characterisations Special Areas of Conservation 1. Abermenai to Aberffraw Dunes SAC 2. Afon Eden - Cors Goch Trawsfynydd SAC 3. Afon Gwyrfrai a Lyn Cwellyn SAC 4. Anglesey Coast: Saltmarsh SAC 5. Anglesey Fens SAC 6. Berwyn and South Clwyd Mountains SAC 7. Cadair Idris SAC 8. Cemlyn Bay SAC 9. Coedydd Aber SAC 10. Cors Fochno SAC 11. Corsydd Eifionydd SAC 12. Glan-traeth SAC 13. Glynllifon SAC 14. Great Orme’s Head SAC 15. Holy Island Coast SAC 16. Llyn Fens SAC 17. Llyen Peninsula and the Sarnau SAC 18. Llyn Dinam SAC 19. Meirionnydd Oakwoods and Bat Sites SAC 20. Menai Strait and Conwy Bay SAC 21. Migneint - Arenig - Dduallt SAC 22. Morfa Harlech a Morfa Dyffryn SAC 23. Preseli SAC 24. Rhinog SAC 25. River Dee and Bala Lake SAC 26. Sea Cliffs of Lleyn SAC 221/A&G JLDP February 2015 1 / 145 ENFUSION Appendix I HRA Anglesey & Gwynedd Joint LDP 27. Snowdonia SAC Special Protection Areas 1. Aberdardon Coast and Bardsey Island SPA 2. Berwyn SPA 3. Craig yr Aderyn SPA 4. Dyfi Estuary SPA 5. Elenydd - Mallaen SPA 6. Holy Island Coast SPA 7. Lavan Sands, Conway Bay SPA 8. Liverpool Bay SPA 9. Migneint - Arenig - Dduallt SPA 10. Mynydd Cilan, Trwyn y Wylfa ac Ynysoedd Sant Tudwal SPA 11. Puffin Island SPA 12. Ynys Feurig, Cemlyn Bay and the Skerries SPA Ramsar 1. Anglesey and Llyn Fens Ramsar 2. Cors Fochno and Dyfi Ramsar 3. Llyn Idwal Ramsar 4. Llyn Tegid Ramsar 221/A&G JLDP February 2015 2 / 145 ENFUSION Appendix I HRA Anglesey & Gwynedd Joint LDP Special Areas of Conservation Abermenai to Aberffraw Dunes SAC Overview The Abermenai to Aberffraw Dunes Special Area of Conservation (SAC) is at the southern end of the Menai Strait in Ynys MÔn and Gwynedd, Wales. -
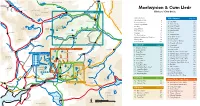
Moelwynion & Cwm Lledr
Llanrwst Llyn Padarn Llyn Ogwen CARNEDDAU Llanberis A5 Llyn Peris B5427 Tryfan Moelwynion & Cwm Lledr A4086 Nant Peris GLYDERAU B5106 Glyder Fach Capel Curig CWM LLEDR MAP PAGE 123 Climbers’ Club Guide Glyder Fawr LLANBERIS PASS A4086 Llynnau Llugwy Mymbyr Introduction A470 Y Moelwynion page 123 The Climbers’ Club 4 Betws-y-Coed Acknowledgments 5 20 Stile Wall 123 B5113 21 Craig Fychan 123 13 Using this guidebook 6 6 Grading 7 22 Craig Wrysgan 123 Carnedd 12 Carnedd Moel-siabod 5 9 10 23 Upper Wrysgan 123 SNOWDON Crag Selector 8 Llyn Llydaw y Cribau 11 3 4 Flora & Fauna 10 24 Moel yr Hydd 123 A470 A5 Rhyd-Ddu Geology 14 25 Pinacl 123 Dolwyddelan Lledr Conwy The Slate Industry 18 26 Waterfall Slab 123 Llyn Gwynant 2 History of Moelwynion Climbing 20 27 Clogwyn yr Oen 123 1 Pentre 8 B4406 28 Carreg Keith 123 Yr Aran -bont Koselig Hour 28 Plas Gwynant Index of Climbs 000 29 Sleep Dancer Buttress 123 7 Penmachno A498 30 Clogwyn y Bustach 123 Cwm Lledr page 30 31 Craig Fach 123 A4085 Bwlch y Gorddinan (Crimea Pass) 1 Clogwyn yr Adar 32 32 Craig Newydd 123 Llyn Dinas MANOD & The TOWN QUARRIES MAP PAGE 123 Machno Ysbyty Ifan 2 Craig y Tonnau 36 33 Craig Stwlan 123 Beddgelert 44 34 Moelwyn Bach Summit Cliffs 123 Moel Penamnen 3 Craig Ddu 38 Y MOELWYNION MAP PAGE 123 35 Moelwyn Bach Summit Quarry 123 Carrog 4 Craig Ystumiau 44 24 29 Cnicht 47 36 Moelwyn Bach Summit Nose 123 Moel Hebog 5 Lone Buttress 48 43 46 B4407 25 30 41 Pen y Bedw Conwy 37 Moelwyn Bach Craig Ysgafn 123 42 Blaenau Ffestiniog 6 Daear Ddu 49 26 31 40 38 38 Craig Llyn Cwm Orthin -

Planning Act 2008 North Wales Wind Farms Connection Project Local
Conwy County Borough Council North Wales Wind Farms Connection Project - Local Impact Report Planning Inspectorate ref: Council ref: EN010014 ENQ/25324 Planning Act 2008 North Wales Wind Farms Connection Project Local Impact Report August 2015 Conwy County Borough Council North Wales Wind Farms Connection Project - Local Impact Report Conwy County Borough Council North Wales Wind Farms Connection Project - Local Impact Report 1. Introduction 1.1 SP Manweb (the Applicant) have applied for a Development Consent Order (DCO) for the installation of a 17km 132kV overground electricity line from a collector station at Clocaenog Forest to a terminal point south of Glascoed Road near St Asaph. The proposed route is partly within the County Borough of Conwy, and partly within the County of Denbighshire. 1.2 The application has been made to the Secretary of State via the Planning Inspectorate under the Planning Act 2008 (the Act). The two local authorities are both consultees in the consent process. 1.3 The Planning Inspectorate strongly encourages local authorities to produce and submit a Local Impact Report (LIR). The definition of an LIR is given in S60(3) of the Act as “a report in writing giving details of the likely impact of the proposed development on the authority’s area (or any part of that area)”. This LIR has been prepared by Conwy County Borough Council (the Council) in accordance with Advice Note 1: Local Impact Reports (Version 2), which was issued by the Planning Inspectorate in April 2012. 1.4 The Council’s Planning Committee endorsed the draft version of the LIR at their Committee meeting on 12th August 2015. -

Dark Sky Reserves Status for Snowdonia Contents
Gwarchodfa Awyr Dywyll Dark Sky Reserve Dark Sky Reserves status for Snowdonia Contents 1. Executive Summary Page 2 2. Introduction to National Parks Page 5 3. Snowdonia National Park Page 6 4. The Problem of Light Pollution Page 11 5. Countering Light Pollution Page 12 6. Letters of Support Page 18 7. The Snowdonia Seeing Stars Initiative’s Anti Light Pollution Strategy Page 19 8. The Proposed IDSR Page 20 9. The Night Sky Quality Survey Page 24 10. The External Lighting Audit Page 28 11. Lighting Management Page 30 12. Communication and Collaboration Page 32 12.1. Media Coverage and Publicity 12.2. Education and Events 12.3. Local Government 13. Lighting Improvements Page 38 14. The Future Page 41 Dark Sky Reserves Snowdonia for status Gwarchodfa Awyr Dywyll Dark Sky Reserve 1.0 Executive Summary This document sets out Snowdonia National Park Authority’s application to the International Dark-Sky Association (IDA) to designate Snowdonia National Park (SNP) as an International Dark Sky Reserve (IDSR). Snowdonia National Park Authority (SNPA) is committed to the protection and conservation of all aspects of the environment, including the night sky, and as such supports the mission and goals of the IDA. The Authority believes that achieving IDSR status for the SNP will further raise the profile of the Light Pollution issue in Wales following the successful application from the Brecon Beacons National Park Authority in 2013. It will assist SNPA in gaining support in protecting the excellent quality of dark skies which we already have in Snowdonia from the general public, business, and politicians, and to improve it further where needed. -

Emmett 2016 STOTEN-15-05214
Spatial patterns and environmental constraints on ecosystem services at a ANGOR UNIVERSITY catchment scale Emmett, Bridget; Cooper, David; Smart, Simon; Jackson, Bethanna; Thomas, Amy; Cosby, Bernard; Evans, Christopher; Glanville, Helen; McDonald, James; Malham, Shelagh; Marshall, Miles; Jarvis, Susan; Rajko-Nenow, Paulina; Webb, Gearoid P.; Ward, Sue; Rowe, Edwin; Jones, Laurence; Vanbergen, Edward J.; Keith, Aidan; Carter, Heather ; Pereira, M. Gloria; Hughes, Steve; Lebron, Inma; Wade, Andrew; Jones, David Science of the Total Environment PRIFYSGOL BANGOR / B DOI: 10.1016/j.scitotenv.2016.04.004 Published: 01/12/2016 Peer reviewed version Cyswllt i'r cyhoeddiad / Link to publication Dyfyniad o'r fersiwn a gyhoeddwyd / Citation for published version (APA): Emmett, B., Cooper, D., Smart, S., Jackson, B., Thomas, A., Cosby, B., Evans, C., Glanville, H., McDonald, J., Malham, S., Marshall, M., Jarvis, S., Rajko-Nenow, P., Webb, G. P., Ward, S., Rowe, E., Jones, L., Vanbergen, E. J., Keith, A., ... Jones, D. (2016). Spatial patterns and environmental constraints on ecosystem services at a catchment scale. Science of the Total Environment, 572, 1586-1600. https://doi.org/10.1016/j.scitotenv.2016.04.004 Hawliau Cyffredinol / General rights Copyright and moral rights for the publications made accessible in the public portal are retained by the authors and/or other copyright owners and it is a condition of accessing publications that users recognise and abide by the legal requirements associated with these rights. • Users may download and print one copy of any publication from the public portal for the purpose of private study or research. • You may not further distribute the material or use it for any profit-making activity or commercial gain • You may freely distribute the URL identifying the publication in the public portal ? Take down policy If you believe that this document breaches copyright please contact us providing details, and we will remove access to the work immediately and investigate your claim. -

Appendix 1 – Water Resource Zone Summaries
Appendix 1 – Water Resource Zone Summaries 2 | P a g e Contents Appendix 1 – Water Resource Zone Summaries .......................................................................................... 1 Contents ..................................................................................................................................................................... 2 Figures ....................................................................................................................................................................... 9 Location Plans .......................................................................................................................................................... 10 Tables ....................................................................................................................................................................... 11 North Eryri/ Ynys Mon - 8001 ....................................................................................................................... 1 North Eryri/ Ynys Mon Water Resources .................................................................................................................... 2 Current Resource Status ............................................................................................................................................ 2 Drought Actions – Sequence of Events ...................................................................................................................... 4 Drought Management Options -

Scoping Opinion Proposed Mynydd Mynyllod Wind Farm
SCOPING OPINION PROPOSED MYNYDD MYNYLLOD WIND FARM, DENBIGHSHIRE AND GWYNEDD, WALES August 2010 independent impartial inclusive CONTENTS EXECUTIVE SUMMARY .................................................................................3 1.0 INTRODUCTION...................................................................................5 Background................................................................................................................. 5 Commission’s Consultation ...................................................................................... 6 Structure of the Document ........................................................................................ 7 2.0 THE PROPOSED DEVELOPMENT .....................................................9 Applicant’s Information ............................................................................................. 9 Commission’s Comment ......................................................................................... 12 3.0 EIA APPROACH AND TOPIC AREAS...............................................17 General Comments on the Scoping Report........................................................... 17 Topic Areas ............................................................................................................... 19 4.0 OTHER INFORMATION ....................................................................31 Appropriate Assessment ......................................................................................... 31 Health Impact Assessment..................................................................................... -

Lake Acidification in the United Kingdom 1800-1986 Evidence from Analysis of Lake Sediments
Lake Acidification in the United Kingdom 1800-1986 Evidence from Analysis of Lake Sediments by R.W. Battarbee N .J. Anderson S. Higgitt F. Oldfield P.G. Appleby V.J. Jones S.T. Patrick R.J. Flower A. Kreiser N.G. Richardson S.C. Fritz M.A.R. Munro B. Rippey E.Y. Haworth J. N atkanski A.C. Stevenson Prepared for the Department of the Environment Under contracts PECD 7/7/139 (UCL) and PECD 7/7/142 (FBA). Palaeoecology Research Unit Department of Geography University College London 26 Bedford Way London WCIH OAP MCMLXXXVIII ENSIS Publishing London Addresses of the authors: R. W. B attarbee Palaeoecology Research Unit, Department of Geography, University College London N.J. Anderson, Department of Ecological Botany, University of Umea. Sweden. P.G. Appleby, Department of Applied Mathematics and Theoretical Physics, University of Liverpool. R.J. Flower, Palaeoecology Research Unit, Department of Geography, University College London. S.C. Fritz, Limnological Research Center, University of Minnesota, USA. E.Y. Haworth, Freshwater Biological Association. Windermere. S. Higgitt, Department of Geography, University of Liverpool. VJ. Jones, Palaeoecology Research Unit, Department of Geography, University College London. A. Kreiser, Palaeoecology Research Unit, Department of Geography, University College London. M.A.R. Munro, Palaeoecology Research Unit, Department of Geography, University College London. J. Natkanski, Palaeoecology Research Unit, Department of Geography, University College London. F. Oldfield, Department of Geography, University of Liverpool. S.T. Patrick, Palaeoecology Research Unit, Department of Geography, University College London. N.G. Richardson, Department of Geography, University of Liverpool. B. Rippey, Freshwater Laboratory, University of Ulster. A.C. -

CORE MANAGEMENT PLAN for Migneint-Arenig-Dduallt SAC/SPA
CYNGOR CEFN GWLAD CYMRU COUNTRYSIDE COUNCIL FOR WALES CORE MANAGEMENT PLAN INCLUDING CONSERVATION OBJECTIVES FOR Migneint-Arenig-Dduallt SAC/SPA Version: F.Evans, N. Young & R.Jenkins Date: 3 March 08 Approved by: NR Thomas 15th April 2008 A Welsh version of all or part of this document can be made available on request. 1 CONTENTS Preface: Purpose of this document Page 1. Vision for the Site................................................................................………….. 3 2. Site Description 2.1 Areas and Designations Covered by this Plan (SAC/SPA)…………… 4 2.2 Outline Description.........................................................................….….. 5 2.3 Outline of Past and Current Management……………………………...5 2.4 Management Units……………………………………………………… 7 3. The Special Features 3.1 Confirmation of Special Features 3.2 Special Features and Management Units 3.3 Conflicts of management..........................................................…………. 9 4. Conservation Objectives Background to Conservation Objectives 4.1 Blanket Bog SAC feature…………………………………………………12 4.2 Dry heath and wet heath SAC features………………………………….17 4.3 Lakes SAC features……………………………………………………….23 4.4 Oak woods SAC feature………………………………………………..…25 4.5 Hen harrier SPA feature………………………………………………….30 4.6 Merlin SPA feature…………………………………………………….….32 4.7 Peregrine SPA feature…………………………………………………….35 5. Assessment of Conservation Status and Management Requirements: 5.1 Blanket bog SAC feature……………………………………………….….37 5.2 Dry heath and wet heath SAC features ……………………………….….38 5.3 Lakes SAC features …………………………………………………….….40 5.4 Oak woods SAC feature……………………………………………………40 5.5 Hen harrier SPA feature…………………………………………………...41 5.6 Merlin SPA feature……………………………………………………..…..42 5.7 Peregrine SPA feature………………………………………………..…….43 6. Action Plan: Summary………………………………………………………………45 7. Glossary 8. References and Annexes PREFACE This document provides the main elements of CCW’s management plan for the site named. -

Conwy Local Development Plan 2007-2022 Adopted October 2013 This Document Is Available to View and Download on the Council’S Web-Site At
Conwy Local Development Plan 2007-2022 Adopted October 2013 This document is available to view and download on the Council’s web-site at: www.conwy.gov.uk/ldp. Copies are also available to view at main libraries and Council offices and can be obtained from the Strategic Planning Policy Service, Muriau Building, Rosehill Street, Conwy LL32 8LD or by telephoning (01492) 575461. If you would like to talk to a planning officer working on the Local Development Plan about any aspect of this document please contact the Strategic Planning Policy Service on (01492) 575181 / 575445 / 575124 / 574232. If you would like an extract or summary of this document on cassette, in large type, in Braille or any other format, please call the Strategic Planning Policy Service on (01492) 575461. “The Conwy Local Development Plan provides a clear vision on how new development can address the challenges we face“ Foreword We are privileged to live and work in a beautiful area. The things we enjoy about Conwy result from the interaction of local communities and the environment. Sometimes features are incidental, sometimes they have evolved over a very long time and sometimes they are planned; whatever the case we need to try and ensure that future communities have the opportunity to enjoy all that is best about Conwy. That is not to say things will remain the same; the area faces many pressures and challenges that can only be addressed by looking at the problems afresh. We must find ways of meeting the local people’s housing and affordable housing needs and enable the provision of well-paid jobs for future communities that are generated by the current and future population, whilst safeguarding the linguistic character of communities, protecting the environment and addressing the threats from flood risk. -

Conwy Management Catchment Summary
Conwy Management Catchment Summary Date Contents 1. Background to the Conwy Management Catchment summary ........................................ 3 2. The Conwy Management Catchment ............................................................................... 4 3. Current Status of the water environment ......................................................................... 7 4. The main challenges ........................................................................................................ 9 5. Objectives and measures .............................................................................................. 11 6. What next? ..................................................................................................................... 16 7. Water Watch Wales ....................................................................................................... 16 Page 2 of 18 www.naturalresourceswales.gov.uk 1. Background to the Conwy Management Catchment summary This management catchment summary supports the current consultation on the updated river basin management plans. Along with detailed information on the Water Watch Wales website, this summary will help to inform and support delivery of local environmental improvements. Natural Resources Wales has adopted the ecosystem approach. This means being more joined up in how we manage the environment and its natural resources to deliver economic, social and environmental benefits for a healthier, more resilient Wales. It means considering and regulating the environment