Cornea/External Disease Preferred Practice Pattern® Development Process and Participants
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Special Considerations in Cataract Surgery: Five Cornea Challenges
Clinical Update EXTRA CONTENT AVAILABLE CATARACT Special Considerations in Cataract Surgery: Five Cornea Challenges by linda roach, contributing writer interviewing preston h. blomquist, md, rosa a. braga-mele, md, kimberly a. drenser, md, phd, herbert e. kaufman, md, marguerite mcdonald, md, and roger f. steinert, md s the most common surgical choices, said Marguerite McDonald, IOL Selection procedure in ophthalmol- MD, of Lynbrook, N.Y. The device en- ogy, replacement of a cloudy ables the surgeon to directly measure 1 crystalline lens with an the eye’s aphakic refractive power in intraocular lens (IOL) usu- the operating room. Aally presents the ophthalmologist with Using intraoperative aberrometry is familiar sets of surgical routines. But becoming more commonplace, as “it what about those cases that involve may help in achieving more accuracy comorbidities or other complicating with IOL power selection,” Dr. Mc- factors? Donald said. Several experts shared their per- Tips on IOL selection. The chosen spectives on approaching out-of-the- IOL should be shaped to neutralize After an off-center LASIK procedure ordinary cataract surgeries in ways spherical aberrations, said Rosa A. such as this, the irregularity of the that offer the best chance at optimiz- Braga-Mele, MD, at the University of corneal topography indicates that a ing patient outcomes. This month, Toronto. “In anybody who has had multifocal IOL should be avoided. here’s a look at five challenges involv- myopic LASIK or PRK, I think it’s very ing the cornea. important to use a negatively aspheric prior to cataract surgery. 2) A dysfunc- IOL, because these patients have more tional, unstable tear film will affect the Challenge: Prior Refractive Surgery positively aberrant corneas. -

Changes in Ocular Rigidityin Endocrine Exophthalmos
Br J Ophthalmol: first published as 10.1136/bjo.42.11.680 on 1 November 1958. Downloaded from Brit. J. Ophthal. (1958) 42, 680. CHANGES IN OCULAR RIGIDITY IN ENDOCRINE EXOPHTHALMOS* BY R. WEEKERS AND G. LAVERGNE From the Ophthalmological Clinic, Lie'ge University Two types of endocrine exophthalmos are frequently distinguished, being referred to thyrotoxic or hyperthyroid exophthalmos, and thyrotropic, ophthahnoplegic, or oedematous exophthalmos. (a) Hyperthyroid or Thyrotoxic Exophthalmos.-This accompanies Graves's disease and is, therefore, much more frequently seen in females than in males. In the majority of cases the exophthalmos is quite unobtrusive or there is merely an appearance of exophthalmos due to retraction of the upper lid. It is associated with a decreased frequency of blinking and a fixed stare. The majority of authors agree that these symptoms should be attributed to an increase in tone of the sym- pathetic system. The importance of the pituitary thyrotropic hormone in thiscopyright. clinical picture is not clear. Hyperthyroid exophthalmos is not complicated either by chemosis or by diplopia, but heterophoria and lack of convergence are often seen. (b) Thyrotropic, Ophthalmoplegic, or Oedematous Exophthalmos.-This may occur either in a subject suffering from verified and treated hyperthyroidism, when the signs of thyrotoxicosis are about to disappear, or in an apparently normal subject free from any thyroid symptom or history of symptoms. The second type http://bjo.bmj.com/ is more frequently seen in males than in females. Thyrotropic exophthalmos is often very marked'and may even lead to irreducible lagophthalmos; it is invariably associated with a disturbance of ocular movements, particularly with elevation of the gaze. -

Eye Disease 1 Eye Disease
Eye disease 1 Eye disease Eye disease Classification and external resources [1] MeSH D005128 This is a partial list of human eye diseases and disorders. The World Health Organisation publishes a classification of known diseases and injuries called the International Statistical Classification of Diseases and Related Health Problems or ICD-10. This list uses that classification. H00-H59 Diseases of the eye and adnexa H00-H06 Disorders of eyelid, lacrimal system and orbit • (H00.0) Hordeolum ("stye" or "sty") — a bacterial infection of sebaceous glands of eyelashes • (H00.1) Chalazion — a cyst in the eyelid (usually upper eyelid) • (H01.0) Blepharitis — inflammation of eyelids and eyelashes; characterized by white flaky skin near the eyelashes • (H02.0) Entropion and trichiasis • (H02.1) Ectropion • (H02.2) Lagophthalmos • (H02.3) Blepharochalasis • (H02.4) Ptosis • (H02.6) Xanthelasma of eyelid • (H03.0*) Parasitic infestation of eyelid in diseases classified elsewhere • Dermatitis of eyelid due to Demodex species ( B88.0+ ) • Parasitic infestation of eyelid in: • leishmaniasis ( B55.-+ ) • loiasis ( B74.3+ ) • onchocerciasis ( B73+ ) • phthiriasis ( B85.3+ ) • (H03.1*) Involvement of eyelid in other infectious diseases classified elsewhere • Involvement of eyelid in: • herpesviral (herpes simplex) infection ( B00.5+ ) • leprosy ( A30.-+ ) • molluscum contagiosum ( B08.1+ ) • tuberculosis ( A18.4+ ) • yaws ( A66.-+ ) • zoster ( B02.3+ ) • (H03.8*) Involvement of eyelid in other diseases classified elsewhere • Involvement of eyelid in impetigo -

Canine Red Eye Elizabeth Barfield Laminack, DVM; Kathern Myrna, DVM, MS; and Phillip Anthony Moore, DVM, Diplomate ACVO
PEER REVIEWED Clinical Approach to the CANINE RED EYE Elizabeth Barfield Laminack, DVM; Kathern Myrna, DVM, MS; and Phillip Anthony Moore, DVM, Diplomate ACVO he acute red eye is a common clinical challenge for tion of the deep episcleral vessels, and is characterized general practitioners. Redness is the hallmark of by straight and immobile episcleral vessels, which run Tocular inflammation; it is a nonspecific sign related 90° to the limbus. Episcleral injection is an external to a number of underlying diseases and degree of redness sign of intraocular disease, such as anterior uveitis and may not reflect the severity of the ocular problem. glaucoma (Figures 3 and 4). Occasionally, episcleral Proper evaluation of the red eye depends on effective injection may occur in diseases of the sclera, such as and efficient diagnosis of the underlying ocular disease in episcleritis or scleritis.1 order to save the eye’s vision and the eye itself.1,2 • Corneal Neovascularization » Superficial: Long, branching corneal vessels; may be SOURCE OF REDNESS seen with superficial ulcerative (Figure 5) or nonul- The conjunctiva has small, fine, tortuous and movable vessels cerative keratitis (Figure 6) that help distinguish conjunctival inflammation from deeper » Focal deep: Straight, nonbranching corneal vessels; inflammation (see Ocular Redness algorithm, page 16). indicates a deep corneal keratitis • Conjunctival hyperemia presents with redness and » 360° deep: Corneal vessels in a 360° pattern around congestion of the conjunctival blood vessels, making the limbus; should arouse concern that glaucoma or them appear more prominent, and is associated with uveitis (Figure 4) is present1,2 extraocular disease, such as conjunctivitis (Figure 1). -
Strabismus: a Decision Making Approach
Strabismus A Decision Making Approach Gunter K. von Noorden, M.D. Eugene M. Helveston, M.D. Strabismus: A Decision Making Approach Gunter K. von Noorden, M.D. Emeritus Professor of Ophthalmology and Pediatrics Baylor College of Medicine Houston, Texas Eugene M. Helveston, M.D. Emeritus Professor of Ophthalmology Indiana University School of Medicine Indianapolis, Indiana Published originally in English under the title: Strabismus: A Decision Making Approach. By Gunter K. von Noorden and Eugene M. Helveston Published in 1994 by Mosby-Year Book, Inc., St. Louis, MO Copyright held by Gunter K. von Noorden and Eugene M. Helveston All rights reserved. No part of this publication may be reproduced, stored in a retrieval system, or transmitted, in any form or by any means, electronic, mechanical, photocopying, recording, or otherwise, without prior written permission from the authors. Copyright © 2010 Table of Contents Foreword Preface 1.01 Equipment for Examination of the Patient with Strabismus 1.02 History 1.03 Inspection of Patient 1.04 Sequence of Motility Examination 1.05 Does This Baby See? 1.06 Visual Acuity – Methods of Examination 1.07 Visual Acuity Testing in Infants 1.08 Primary versus Secondary Deviation 1.09 Evaluation of Monocular Movements – Ductions 1.10 Evaluation of Binocular Movements – Versions 1.11 Unilaterally Reduced Vision Associated with Orthotropia 1.12 Unilateral Decrease of Visual Acuity Associated with Heterotropia 1.13 Decentered Corneal Light Reflex 1.14 Strabismus – Generic Classification 1.15 Is Latent Strabismus -

Eye Care in the Intensive Care Unit (ICU)
Ophthalmic Services Guidance Eye Care in the Intensive Care Unit (ICU) June 2017 18 Stephenson Way, London, NW1 2HD T. 020 7935 0702 [email protected] rcophth.ac.uk @RCOphth © The Royal College of Ophthalmologists 2017 All rights reserved For permission to reproduce any of the content contained herein please contact [email protected] Contents Section page 1 Summary 3 2 Introduction 3 Protecting the eye of the vulnerable patient 4 3 Identifying disease of the eye 6 Exposure keratopathy and corneal abrasion 6 Chemosis 8 Microbial infections 8 4 Rare eye conditions in ICU 10 Red eye in a septic patient: possible endogenous endophthalmitis 10 Other problems 11 5 Delivering treatment to the eye when it is prescribed 11 Red eye in ICU patient 12 6. Systemic fungal infection and the eye for intensivists 14 7. Tips for ophthalmologists seeing patients in ICU 14 8. Authors 16 9. References 17 Date of review: July 2020 2017/PROF/350 2 1 Summary This document aims to provide advice and information for clinical staff who are involved in eye care in the ICU. It is primarily intended to help non-ophthalmic ICU staff to: 1. protect the eye in vulnerable patients, thus preventing ICU-related eye problems 2. identify disease affecting the eye in ITU patients, and specifically those which might need ophthalmic referral 3. deliver treatment to the eye when it is prescribed It concentrates primarily on the common problems of the eye surface but also touches on other less common conditions. As such, it should also be helpful to those ophthalmologists asked for advice about ICU patients. -

Pulsatile Proptosis
Pulsatile Proptosis Julia Elpers, MD Grand Rounds January 10th, 2020 Department of Ophthalmology and Visual Sciences Patient Presentation CC Red, proptotic eye HPI Consult from neurosurgery for eye injury with proptosis in a 74 yo WF who fell down a flight of stairs. She sustained many bodily fractures, facial and skull fractures, and subarachnoid hemorrhage. She is now intubated and sedated in the ICU. History Squamous Cell Carcinoma Past Medical History Migraine Family Hx Noncontributory Meds Sumatriptan, ASA 81mg Allergies Sulfa -Never Smoker Social Hx -1 drink per day alcohol -No illicits RoS Unable to obtain Physical Exam OD OS VAscD Unable to Obtain Unable to Obtain Pupils 3+ RAPD 4→3mm IOP 55 mmHg 14 mmHg EOM Unable to Obtain Unable to Obtain CVF Unable to Obtain Unable to Obtain Lids Ecchymosis, edema Ecchymosis, edema External Exam Physical Exam SLE OD OS 4+ chemosis, injection C/S 1+chemosis, injection with corkscrew vessels K Hazy Clear AC Formed Formed Iris Flat Flat Lens 1+NS 1+ NS Vit Clear Clear Fundus OD OS Optic Nerve +1 edema, slight pallor C/D 0.5, pink and sharp Retinal whitening with blunted foveal reflex, rare Macula Cherry red spot intraretinal heme Attenuated arteries, Vessels Dilated veins dilated veins Exudates around superior Periphery attached arcade Imaging – CT head Assessment • 74 yo WF intubated and sedated after a fall down flight of stairs sustaining skull and facial fractures and subarachnoid hemorrhage, now with pulsatile proptosis, severe injection and chemosis, RAPD, and retinal whitening with cherry red. • Concerning for Carotid Cavernous Fistula and Central Retinal Artery Occlusion • Differential Diagnosis of pulsatile proptosis • CCF fistula • Normal intracranial pulsation transmitted to the orbit due to skull base fracture Plan • Informed neurosurgery that clinically she appears to have C-C fistula and recommend neurosurgical intervention. -

The Definition and Classification of Dry Eye Disease
DEWS Definition and Classification The Definition and Classification of Dry Eye Disease: Report of the Definition and Classification Subcommittee of the International Dry E y e W ork Shop (2 0 0 7 ) ABSTRACT The aim of the DEWS Definition and Classifica- I. INTRODUCTION tion Subcommittee was to provide a contemporary definition he Definition and Classification Subcommittee of dry eye disease, supported within a comprehensive clas- reviewed previous definitions and classification sification framework. A new definition of dry eye was devel- T schemes for dry eye, as well as the current clinical oped to reflect current understanding of the disease, and the and basic science literature that has increased and clarified committee recommended a three-part classification system. knowledge of the factors that characteriz e and contribute to The first part is etiopathogenic and illustrates the multiple dry eye. Based on its findings, the Subcommittee presents causes of dry eye. The second is mechanistic and shows how herein an updated definition of dry eye and classifications each cause of dry eye may act through a common pathway. based on etiology, mechanisms, and severity of disease. It is stressed that any form of dry eye can interact with and exacerbate other forms of dry eye, as part of a vicious circle. II. GOALS OF THE DEFINITION AND Finally, a scheme is presented, based on the severity of the CLASSIFICATION SUBCOMMITTEE dry eye disease, which is expected to provide a rational basis The goals of the DEWS Definition and Classification for therapy. These guidelines are not intended to override the Subcommittee were to develop a contemporary definition of clinical assessment and judgment of an expert clinician in dry eye disease and to develop a three-part classification of individual cases, but they should prove helpful in the conduct dry eye, based on etiology, mechanisms, and disease stage. -

Update on Ocular Leprosy
Ocular Leprosy Report Update on Ocular Leprosy Gordon J Johnson closed and leprosy workers are being MD FRCS FRCOphth phased out or re-deployed. Tamil Nadu is Professor and Director the first state in India in which leprosy con- International Centre for Eye Health trol has become fully integrated into the Institute of Ophthalmology general health services; other countries are following the same pattern. Under these 11-43 Bath Street The faces of leprosy conditions there is a real danger that new London EC1V 9EL, UK Photos: Margreet Hogeweg cases will be missed, and disabilities will Numbers of People with Leprosy not be adequately dealt with. Therefore, 1. At time of diagnosis guidelines for the responsibilities and The setting in which the decision to The widespread use of Multiple Drug training of general health workers must treat will be made in India will be the Therapy (MDT) in leprosy control pro- be rapidly developed. The eye care pro- Primary Health Centre (PHC), under grammes has resulted in a great reduction gramme must also assume great responsi- the supervision of the PHC Medical in worldwide prevalence. There is a mixed bility. Officer (PHC MO). Each centre may picture from country to country, so that Some of the problems and opportunities only see 5Ð10 new patients a year, of there is still a high incidence of newly associated with integration of leprosy care whom only one may have lagophthal- diagnosed cases in some regions, for exam- into Primary Health Care were identified at mos. ple in northern Brazil and parts of India. -

Update on Thyroid-Associated Ophthalmopathy with a Special Emphasis on the Ocular Surface Priscila Novaes1†, Ana Beatriz Diniz Grisolia1† and Terry J
Novaes et al. Clinical Diabetes and Endocrinology (2016) 2:19 DOI 10.1186/s40842-016-0037-5 REVIEWARTICLE Open Access Update on thyroid-associated Ophthalmopathy with a special emphasis on the ocular surface Priscila Novaes1†, Ana Beatriz Diniz Grisolia1† and Terry J. Smith1,2,3* Abstract Thyroid-associated ophthalmopathy (TAO) is a condition associated with a wide spectrum of ocular changes, usually in the context of the autoimmune syndrome, Graves’ disease. In this topical review, we attempted to provide a roadmap of the recent advances in current understanding the pathogenesis of TAO, important aspects of its clinical presentation, its impact on the ocular surface, describe the tissue abnormalities frequently encountered, and describe how TAO is managed today. We also briefly review how increased understanding of the disease should culminate in improved therapies for patients with this vexing condition. Keywords: Graves’ disease, Ophthalmopathy, Thyroid, Orbit, Ocular surface Background factors, and several partially characterized environmental Thyroid-associated ophthalmopathy (TAOa, aka thyroid triggers [8]. Several recent reviews have addressed TAO; eye disease or Graves’ ophthalmopathy) refers to several however none has detailed insights of the association ocular manifestations related to the systemic auto- and interactions between TAO, the ocular surface (OS) immune process, Graves’ disease (GD) [1]. This syn- and dry eye syndrome (DES). We have thus included an drome has been attributed to the loss of immune emphasis on the OS and DES in this review. tolerance to the thyrotropin receptor (TSHR) and per- haps other auto-antigenic proteins [2, 3]. TAO results Pathogenesis from the linked conspiracy of auto-reactivity and tissue The central participant in the pathogenesis of TAO is remodeling. -

CORNEAL ULCERS Diagnosis and Management
CORNEAL ULCERS Diagnosis and Management System requirement: • Windows XP or above • Power DVD player (Software) • Windows Media Player 10.0 version or above • Quick time player version 6.5 or above Accompanying DVD ROM is playable only in Computer and not in DVD player. Kindly wait for few seconds for DVD to autorun. If it does not autorun then please do the following: • Click on my computer • Click the drive labelled JAYPEE and after opening the drive, kindly double click the file Jaypee CORNEAL ULCERS Diagnosis and Management Namrata Sharma MD DNB MNAMS Associate Professor of Ophthalmology Cornea, Cataract and Refractive Surgery Services Dr. Rajendra Prasad Centre for Ophthalmic Sciences All India Institute of Medical Sciences, New Delhi India Rasik B Vajpayee MS FRCSEd FRANZCO Head, Corneal and Cataract Surgery Centre for Eye Research Australia Royal Victorian Eye and Ear Hospital University of Melbourne Australia Forewords Hugh R Taylor Peter R Laibson ® JAYPEE BROTHERS MEDICAL PUBLISHERS (P) LTD New Delhi • Ahmedabad • Bengaluru • Chennai • Hyderabad • Kochi • Kolkata • Lucknow • Mumbai • Nagpur Published by Jitendar P Vij Jaypee Brothers Medical Publishers (P) Ltd B-3 EMCA House, 23/23B Ansari Road, Daryaganj New Delhi 110 002, India Phones: +91-11-23272143, +91-11-23272703, +91-11-23282021, +91-11-23245672 Rel: +91-11-32558559, Fax: +91-11-23276490, +91-11-23245683 e-mail: [email protected] Visit our website: www.jaypeebrothers.com Branches • 2/B, Akruti Society, Jodhpur Gam Road Satellite Ahmedabad 380 015, Phones: +91-79-26926233, -
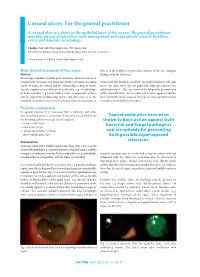
Corneal Ulcers: for the General Practitioner
Corneal ulcers: For the general practitioner A corneal ulcer is a defect in the epithelial layer of the cornea. e general practitioner may play an important role in early management and appropriate referral. Incidence varies and depends on aetiology. S Ballim, MB ChB, Dip Ophth (SA), FC Ophth (SA) Department of Ophthalmology, University of KwaZulu-Natal, Durban, South Africa Correspondence to: S Ballim ([email protected]) Basic clinical assessment of the cornea clue as to the health of the posterior segment of the eye. Compare History ndings with the fellow eye. Presenting complaints include pain, irritation, decreased vision or foreign-body sensation. Ask about any history of trauma, including Fluorescein dye should be instilled. Any epithelial defects will stain nature of injury, the timing and the relationship to drop in vision. green. e stain shows up best under blue light (present on some Specic enquiry as to instillation of medication (e.g. steroid drops) ophthalmoscopes). is can, however, be adequately demonstrated or home remedies (e.g. breast milk or urine) is important, as these with a standard torch. e tear lm will become apparent and the may be important predisposing factors and give clues as to the lacus lacrimalis can be assessed. Dry eye is a very signicant factor aetiology. Contact lens use is a very common cause of corneal ulcers. in many corneal epithelial disorders. Tools for examination In general practice it is presumed that a slit-lamp and other specialised equipment is unavailable. It would be reasonable to have Topical antibiotics have been the following ophthalmic tools at one’s disposal: shown to have action against both • visual acuity chart bacterial and fungal pathogens • uorescein strips • topical anaesthetic eye drops and are suitable for preventing • direct ophthalmoscope.