Oncogenic Features of Neuromedin U in Breast Cancer Are Associated with NMUR2 Expression Involving Crosstalk with Members of the WNT Signaling Pathway
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Neuromedin U Directly Stimulates Growth of Cultured Rat Calvarial Osteoblast-Like Cells Acting Via the NMU Receptor 2 Isoform
363-368 1/8/08 15:53 Page 363 INTERNATIONAL JOURNAL OF MOLECULAR MEDICINE 22: 363-368, 2008 363 Neuromedin U directly stimulates growth of cultured rat calvarial osteoblast-like cells acting via the NMU receptor 2 isoform MARCIN RUCINSKI, AGNIESZKA ZIOLKOWSKA, MARIANNA TYCZEWSKA, MARTA SZYSZKA and LUDWIK K. MALENDOWICZ Department of Histology and Embryology, Poznan University of Medical Sciences, 6 Swiecicki St., 60-781 Poznan, Poland Received April 4, 2008; Accepted June 2, 2008 DOI: 10.3892/ijmm_00000031 Abstract. The neuromedin U (NMU) system is composed of nervous system. Among others, peptides involved in regulation NMU, neuromedin S (NMS) and their receptors NMUR1 and of energy homeostasis belong to this group of compounds NMUR2. This system is involved in the regulation of energy (1-3), and the best recognised is leptin, an adipocyte-derived homeostasis, neuroendocrine functions, immune response, anorexigenic hormone, which plays a role in regulating bone circadian rhythm and spermatogenesis. The present study formation. Acting directly this pleiotropic cytokine exerts a aimed to investigate the possible role of the NMU system in stimulatory effect on bone formation. While acting through regulating functions of cultured rat calvarial osteoblast-like the central nervous system (CNS) leptin suppresses bone (ROB) cells. By using QPCR, high expression of NMU formation (4-10). Moreover, OB-Rb mRNA is expressed in mRNA was found in freshly isolated ROB cells while after 7, osteoblasts, and in vitro leptin enhances their proliferation 14, and 21 days of culture, expression of the studied gene and has no effect on osteocalcin and osteopontin production by was very low. -

Molecular Dissection of G-Protein Coupled Receptor Signaling and Oligomerization
MOLECULAR DISSECTION OF G-PROTEIN COUPLED RECEPTOR SIGNALING AND OLIGOMERIZATION BY MICHAEL RIZZO A Dissertation Submitted to the Graduate Faculty of WAKE FOREST UNIVERSITY GRADUATE SCHOOL OF ARTS AND SCIENCES in Partial Fulfillment of the Requirements for the Degree of DOCTOR OF PHILOSOPHY Biology December, 2019 Winston-Salem, North Carolina Approved By: Erik C. Johnson, Ph.D. Advisor Wayne E. Pratt, Ph.D. Chair Pat C. Lord, Ph.D. Gloria K. Muday, Ph.D. Ke Zhang, Ph.D. ACKNOWLEDGEMENTS I would first like to thank my advisor, Dr. Erik Johnson, for his support, expertise, and leadership during my time in his lab. Without him, the work herein would not be possible. I would also like to thank the members of my committee, Dr. Gloria Muday, Dr. Ke Zhang, Dr. Wayne Pratt, and Dr. Pat Lord, for their guidance and advice that helped improve the quality of the research presented here. I would also like to thank members of the Johnson lab, both past and present, for being valuable colleagues and friends. I would especially like to thank Dr. Jason Braco, Dr. Jon Fisher, Dr. Jake Saunders, and Becky Perry, all of whom spent a great deal of time offering me advice, proofreading grants and manuscripts, and overall supporting me through the ups and downs of the research process. Finally, I would like to thank my family, both for instilling in me a passion for knowledge and education, and for their continued support. In particular, I would like to thank my wife Emerald – I am forever indebted to you for your support throughout this process, and I will never forget the sacrifices you made to help me get to where I am today. -

Expression of Neuromedins S and U and Their Receptors in the Hypothalamus and Endocrine Glands of the Rat
255-259 4/7/07 20:57 Page 255 INTERNATIONAL JOURNAL OF MOLECULAR MEDICINE 20: 255-259, 2007 255 Expression of neuromedins S and U and their receptors in the hypothalamus and endocrine glands of the rat MARCIN RUCINSKI1, AGNIESZKA ZIOLKOWSKA1, GIULIANO NERI2, MARCIN TREJTER1, TOMASZ ZEMLEDUCH1, MARIANNA TYCZEWSKA1, GASTONE G. NUSSDORFER2 and LUDWIK K. MALENDOWICZ1 1Department of Histology and Embryology, Poznan School of Medicine, Poznan PL-69781, Poland; 2Department of Human Anatomy and Physiology, University of Padua, I-35121 Padua, Italy Received March 14, 2007; Accepted April 20, 2007 Abstract. Neuromedin S (NMS) and neuromedin U (NMU) NMS because it is highly expressed in the hypothalamic are regulatory peptides that share the C-terminal amino-acid suprachiasmatic nucleus (7-9). sequence and act via common G protein-coupled receptors Available findings stress numerous differences between called NMUR1 and NMUR2. Semiquantitative real time-PCR NMS and NMU. NMS is a neuropeptide mainly involved in showed that in the rat hypothalamus and testis NMS gene the modulation of the immune response, regulation of circadian expression was markedly higher than that of the NMU gene, rhythms and spermatogenesis. Moreover, NMS has been also while the reverse occurred in the anterior pituitary and thyroid suggested to be a potent anorexigenic hormone that acts, gland. Low expression of both genes was detected in the among others, via CRH neurons (7-9). NMU is a brain-gut thymus, adrenal gland and ovary, whereas in the pancreatic peptide involved in the regulation of energy homeostasis and islets only the expression of NMU mRNA was detected. In neuroendocrine functions (4,5,10-18). -

ILC2 Activation by Protozoan Commensal Microbes
International Journal of Molecular Sciences Review ILC2 Activation by Protozoan Commensal Microbes Kyle Burrows 1 , Louis Ngai 1 , Flora Wong 1,2, David Won 1 and Arthur Mortha 1,* 1 University of Toronto, Department of Immunology, Toronto, ON M5S 1A8, Canada; [email protected] (K.B.) [email protected] (L.N.); fl[email protected] (F.W.); [email protected] (D.W.) 2 Ranomics, Inc. Toronto, ON M5G 1X5, Canada * Correspondence: [email protected] Received: 3 September 2019; Accepted: 27 September 2019; Published: 30 September 2019 Abstract: Group 2 innate lymphoid cells (ILC2s) are a member of the ILC family and are involved in protective and pathogenic type 2 responses. Recent research has highlighted their involvement in modulating tissue and immune homeostasis during health and disease and has uncovered critical signaling circuits. While interactions of ILC2s with the bacterial microbiome are rather sparse, other microbial members of our microbiome, including helminths and protozoans, reveal new and exciting mechanisms of tissue regulation by ILC2s. Here we summarize the current field on ILC2 activation by the tissue and immune environment and highlight particularly new intriguing pathways of ILC2 regulation by protozoan commensals in the intestinal tract. Keywords: ILC2; protozoa; Trichomonas; Tritrichomonas musculis; mucosal immunity; taste receptors; succinate; intestinal immunity; type 2 immunity; commensals 1. The ILC Lineage 1.1. The Family of Innate Lymphoid Cells Research over the last decade has redirected focus away from classical immune cell interactions within lymphoid tissues towards immunity within non-lymphoid tissues. Within these tissues, immune interactions involve local adaptation and rapid responses by tissue-resident immune cells. -

Neural Regulation of Interactions Between Group 2 Innate Lymphoid Cells and Pulmonary Immune Cells
Prime Archives in Immunology: 2nd Edition Book Chapter Neural Regulation of Interactions between Group 2 Innate Lymphoid Cells and Pulmonary Immune Cells Weiwei Chen1,2, Qiang Shu2 and Jie Fan1,3,4* 1Department of Surgery, University of Pittsburgh School of Medicine, USA 2The Children‟s Hospital, Zhejiang University School of Medicine, National Clinical Research Center for Child Health, China 3Research and Development, Veterans Affairs Pittsburgh Healthcare System, USA 4McGowan Institute for Regenerative Medicine, University of Pittsburgh, USA *Corresponding Author: Jie Fan, Department of Surgery, University of Pittsburgh School of Medicine, Pittsburgh, PA, USA Published January 18, 2021 This Book Chapter is a republication of an article published by Jie Fan, et al. at Frontiers in Immunology in October 2020. (Chen W, Shu Q and Fan J (2020) Neural Regulation of Interactions Between Group 2 Innate Lymphoid Cells and Pulmonary Immune Cells. Front. Immunol. 11:576929. doi: 10.3389/fimmu.2020.576929) How to cite this book chapter: Weiwei Chen, Qiang Shu, Jie Fan. Neural Regulation of Interactions between Group 2 Innate Lymphoid Cells and Pulmonary Immune Cells. In: Ajmal Khan, Ahmed Al-Harrasi, editors. Prime Archives in Immunology: 2nd Edition. Hyderabad, India: Vide Leaf. 2021. 1 www.videleaf.com Prime Archives in Immunology: 2nd Edition © The Author(s) 2021. This article is distributed under the terms of the Creative Commons Attribution 4.0 International License(http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited. Author Contributions: WC collected the data and drafted the manuscript. WC, QS, and JF conceived and designed the study. -

Guthrie Cdna Resource Center
cDNA Resource Center cDNA Resource Center Catalog cDNA Resource Center Missouri University of Science and Technology 400 W 11th Rolla, MO 65409 TEL: (573) 341-7610 FAX: (573) 341-7609 EMAIL: [email protected] www.cdna.org September, 2008 1 cDNA Resource Center Visit our web site for product updates 2 cDNA Resource Center The cDNA Resource Center The cDNA Resource Center is a service provided by the faculty of the Department of Biological Sciences of Missouri University of Science and Technology. The purpose of the cDNA Resource Center is to further scientific investigation by providing cDNA clones of human proteins involved in signal transduction processes. This is achieved by providing high quality clones for important signaling proteins in a timely manner. By high quality, we mean that the clones are • Sequence verified • Propagated in a versatile vector useful in bacterial and mammalian systems • Free of extraneous 3' and 5' untranslated regions • Expression verified (in most cases) by coupled in vitro transcription/translation assays • Available in wild-type, epitope-tagged and common mutant forms (e.g., constitutively- active or dominant negative) By timely, we mean that the clones are • Usually shipped within a day from when you place your order. Clones can be ordered from our web pages, by FAX or by phone. Within the United States, clones are shipped by overnight courier (FedEx); international orders are shipped International Priority (FedEx). The clones are supplied for research purposes only. Details on use of the material are included on the Material Transfer Agreement (page 3). Clones are distributed by agreement in Invitrogen's pcDNA3.1+ vector. -

Supplementary Table S4. FGA Co-Expressed Gene List in LUAD
Supplementary Table S4. FGA co-expressed gene list in LUAD tumors Symbol R Locus Description FGG 0.919 4q28 fibrinogen gamma chain FGL1 0.635 8p22 fibrinogen-like 1 SLC7A2 0.536 8p22 solute carrier family 7 (cationic amino acid transporter, y+ system), member 2 DUSP4 0.521 8p12-p11 dual specificity phosphatase 4 HAL 0.51 12q22-q24.1histidine ammonia-lyase PDE4D 0.499 5q12 phosphodiesterase 4D, cAMP-specific FURIN 0.497 15q26.1 furin (paired basic amino acid cleaving enzyme) CPS1 0.49 2q35 carbamoyl-phosphate synthase 1, mitochondrial TESC 0.478 12q24.22 tescalcin INHA 0.465 2q35 inhibin, alpha S100P 0.461 4p16 S100 calcium binding protein P VPS37A 0.447 8p22 vacuolar protein sorting 37 homolog A (S. cerevisiae) SLC16A14 0.447 2q36.3 solute carrier family 16, member 14 PPARGC1A 0.443 4p15.1 peroxisome proliferator-activated receptor gamma, coactivator 1 alpha SIK1 0.435 21q22.3 salt-inducible kinase 1 IRS2 0.434 13q34 insulin receptor substrate 2 RND1 0.433 12q12 Rho family GTPase 1 HGD 0.433 3q13.33 homogentisate 1,2-dioxygenase PTP4A1 0.432 6q12 protein tyrosine phosphatase type IVA, member 1 C8orf4 0.428 8p11.2 chromosome 8 open reading frame 4 DDC 0.427 7p12.2 dopa decarboxylase (aromatic L-amino acid decarboxylase) TACC2 0.427 10q26 transforming, acidic coiled-coil containing protein 2 MUC13 0.422 3q21.2 mucin 13, cell surface associated C5 0.412 9q33-q34 complement component 5 NR4A2 0.412 2q22-q23 nuclear receptor subfamily 4, group A, member 2 EYS 0.411 6q12 eyes shut homolog (Drosophila) GPX2 0.406 14q24.1 glutathione peroxidase -

An Evolutionary Based Strategy for Predicting Rational Mutations in G Protein-Coupled Receptors
Ecology and Evolutionary Biology 2021; 6(3): 53-77 http://www.sciencepublishinggroup.com/j/eeb doi: 10.11648/j.eeb.20210603.11 ISSN: 2575-3789 (Print); ISSN: 2575-3762 (Online) An Evolutionary Based Strategy for Predicting Rational Mutations in G Protein-Coupled Receptors Miguel Angel Fuertes*, Carlos Alonso Department of Microbiology, Centre for Molecular Biology “Severo Ochoa”, Spanish National Research Council and Autonomous University, Madrid, Spain Email address: *Corresponding author To cite this article: Miguel Angel Fuertes, Carlos Alonso. An Evolutionary Based Strategy for Predicting Rational Mutations in G Protein-Coupled Receptors. Ecology and Evolutionary Biology. Vol. 6, No. 3, 2021, pp. 53-77. doi: 10.11648/j.eeb.20210603.11 Received: April 24, 2021; Accepted: May 11, 2021; Published: July 13, 2021 Abstract: Capturing conserved patterns in genes and proteins is important for inferring phenotype prediction and evolutionary analysis. The study is focused on the conserved patterns of the G protein-coupled receptors, an important superfamily of receptors. Olfactory receptors represent more than 2% of our genome and constitute the largest family of G protein-coupled receptors, a key class of drug targets. As no crystallographic structures are available, mechanistic studies rely on the use of molecular dynamic modelling combined with site-directed mutagenesis data. In this paper, we hypothesized that human-mouse orthologs coding for G protein-coupled receptors maintain, at speciation events, shared compositional structures independent, to some extent, of their percent identity as reveals a method based in the categorization of nucleotide triplets by their gross composition. The data support the consistency of the hypothesis, showing in ortholog G protein-coupled receptors the presence of emergent shared compositional structures preserved at speciation events. -

Neuropeptides Controlling Energy Balance: Orexins and Neuromedins
Neuropeptides Controlling Energy Balance: Orexins and Neuromedins Joshua P. Nixon, Catherine M. Kotz, Colleen M. Novak, Charles J. Billington, and Jennifer A. Teske Contents 1 Brain Orexins and Energy Balance ....................................................... 79 1.1 Orexin............................................................................... 79 2 Orexin and Feeding ....................................................................... 80 3 Orexin and Arousal ....................................................................... 83 J.P. Nixon • J.A. Teske Veterans Affairs Medical Center, Research Service (151), Minneapolis, MN, USA Department of Food Science and Nutrition, University of Minnesota, 1334 Eckles Avenue, St. Paul, MN 55108, USA Minnesota Obesity Center, University of Minnesota, 1334 Eckles Avenue, St. Paul, MN 55108, USA C.M. Kotz (*) Veterans Affairs Medical Center, GRECC (11 G), Minneapolis, MN, USA Veterans Affairs Medical Center, Research Service (151), Minneapolis, MN, USA Department of Food Science and Nutrition, University of Minnesota, 1334 Eckles Avenue, St. Paul, MN 55108, USA Minnesota Obesity Center, University of Minnesota, 1334 Eckles Avenue, St. Paul, MN 55108, USA e-mail: [email protected] C.M. Novak Department of Biological Sciences, Kent State University, Kent, OH, USA C.J. Billington Veterans Affairs Medical Center, Research Service (151), Minneapolis, MN, USA Veterans Affairs Medical Center, Endocrine Unit (111 G), Minneapolis, MN, USA Department of Food Science and Nutrition, University of Minnesota, 1334 Eckles Avenue, St. Paul, MN 55108, USA Minnesota Obesity Center, University of Minnesota, 1334 Eckles Avenue, St. Paul, MN 55108, USA H.-G. Joost (ed.), Appetite Control, Handbook of Experimental Pharmacology 209, 77 DOI 10.1007/978-3-642-24716-3_4, # Springer-Verlag Berlin Heidelberg 2012 78 J.P. Nixon et al. 4 Orexin Actions on Endocrine and Autonomic Systems ................................. 84 5 Orexin, Physical Activity, and Energy Expenditure .................................... -
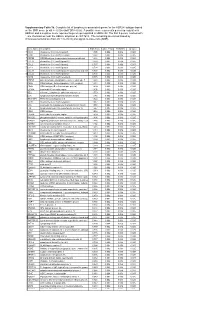
* Supplementary Table 3B. Complete List of Lymphocytic-Associated Genes
Supplementary Table 3b. Complete list of lymphocytic-associated genes for the HER2+I subtype based on the SNR score (p-val <= 0.005 and FDR<=0.05). A positive score represents genes up-regulated in HER2+I and a negative score represents genes up-regulated in HER2+NI. The first 9 genes, marked with *, are chemokines near the HER2+ amplicon at chr17q12. The remaining are sorted based by chromosomal location (from chr 1 to chr X) and signal-to-noise ratio (SNR). Gene Name Description SNR Score Feature P value FDR(BH) Q Value * CCL5 chemokine (C-C motif) ligand 5 1.395 0.002 0.036 0.025 * CCR7 chemokine (C-C motif) receptor 7 1.362 0.002 0.036 0.025 * CD79B CD79B antigen (immunoglobulin-associated beta) 1.248 0.002 0.036 0.025 * CCL13 chemokine (C-C motif) ligand 13 1.003 0.002 0.036 0.025 * CCL2 chemokine (C-C motif) ligand 2 0.737 0.004 0.056 0.037 * CCL8 chemokine (C-C motif) ligand 8 0.724 0.002 0.036 0.025 * CCL18 chemokine (C-C motif) ligand 18 (pulmonary and activ 0.703 0.002 0.036 0.025 * CCL23 chemokine (C-C motif) ligand 23 0.701 0.002 0.036 0.025 * CCR6 chemokine (C-C motif) receptor 6 0.715 0.002 0.036 0.025 PTPN7 protein tyrosine phosphatase, non-receptor type 7 1.861 0.002 0.036 0.025 CD3Z CD3Z antigen, zeta polypeptide (TiT3 complex) 1.811 0.002 0.036 0.025 CD48 CD48 antigen (B-cell membrane protein) 1.809 0.002 0.036 0.025 IL10RA interleukin 10 receptor, alpha 1.806 0.002 0.036 0.025 SELL selectin L (lymphocyte adhesion molecule 1) 1.773 0.002 0.036 0.025 LCK lymphocyte-specific protein tyrosine kinase 1.745 0.002 0.036 0.025 -

Neuromedin U: a Small Peptide in the Big World of Cancer
cancers Review Neuromedin U: A Small Peptide in the Big World of Cancer Patrycja Przygodzka 1,* , Kamila Soboska 1,2, Ewelina Sochacka 1,2 and Joanna Boncela 1,* 1 Institute of Medical Biology, Polish Academy of Sciences, 106 Lodowa Str, 93-232 Lodz, Poland 2 Faculty of Biology and Environmental Protection, University of Lodz, 141/143 Pomorska Str, 90-236 Lodz, Poland * Correspondence: [email protected] or [email protected] (P.P.); [email protected] (J.B.) Received: 29 July 2019; Accepted: 3 September 2019; Published: 5 September 2019 Abstract: Neuromedin U (NMU), a neuropeptide isolated from porcine spinal cord and named because of its activity as a rat uterus smooth muscle contraction inducer, is emerging as a new player in the tumorigenesis and/or metastasis of many types of cancers. Expressed in a variety of tissues, NMU has been shown to possess many important activities in the central nervous system as well as on the periphery. Along with the main structural and functional features of NMU and its currently known receptors, we summarized a growing number of recently published data from different tissues and cells that associate NMU activity with cancer development and progression. We ask if, based on current reports, NMU can be included as a marker of these processes and/or considered as a therapeutic target. Keywords: Neuromedin U; NMU; GPCR receptors; cancer 1. Neuromedin U Structure: Implications for Biological Activity Since it was identified in 1985, neuromedin U (NMU) expression has been found in many species in various isoforms. The longer forms NMU-17 (toad), NMU-21 (goldfish), NMU-23 (rat, tree frog), NMU-25 (human, pig, rabbit, dog, chicken, frog, goldfish), and the shorter forms NMU-8 (pig, dog) and NMU-9 (guinea pig, chicken) have been identified (reviewed in Reference [1]). -

Neuromedins U and S Involvement in the Regulation of the Hypothalamo–Pituitary–Adrenal Axis
REVIEW ARTICLE published: 05 December 2012 doi: 10.3389/fendo.2012.00156 Neuromedins U and S involvement in the regulation of the hypothalamo–pituitary–adrenal axis Ludwik K. Malendowicz*, Agnieszka Ziolkowska and Marcin Rucinski Department of Histology and Embryology, Poznan University of Medical Sciences, Poznan, Poland Edited by: We reviewed neuromedin U (NMU) and neuromedin S (NMS) involvement in the regulation Hubert Vaudry, University of Rouen, of the hypothalamo–pituitary–adrenal (HPA) axis function. NMU and NMS are structurally France related and highly conserved neuropeptides. They exert biological effects via two GPCR Reviewed by: receptors designated as NMUR1 and NMUR2 which show differential expression. NMUR1 James A. Carr, Texas Tech University, USA is expressed predominantly at the periphery, while NMUR2 in the central nervous system. Gábor B. Makara, Hungarian Elements of the NMU/NMS and their receptors network are also expressed in the HPA Academy of Sciences, Hungary axis and progress in molecular biology techniques provided new information on their *Correspondence: actions within this system. Several lines of evidence suggest that within the HPA axis Ludwik K. Malendowicz, NMU and NMS act at both hypothalamic and adrenal levels. Moreover, new data suggest Department of Histology and Embryology, Poznan University of that NMU and NMS are involved in central and peripheral control of the stress response. Medical Sciences, 6 Swie¸cickiSt., 60-781 Poznan, Poland. Keywords: neuromedin U, neuromedin S, hypothalamus, pituitary, adrenal e-mail: [email protected] INTRODUCTION Identification of specific NMU receptors (NMUR1 and In search for new biologically active peptides, the group of NMUR2) and its anorexigenic action have enhanced interest in Minamino, Kangawa, and Matsuo in the 1980s isolated numerous physiological role of NMU and NMS (Howard et al., 2000; Ida small neuropeptides from porcine spinal cord.