Mating Patterns and Genetic Diversity in the Wild Daffodil Narcissus Longispathus (Amaryllidaceae)
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Conserving Europe's Threatened Plants
Conserving Europe’s threatened plants Progress towards Target 8 of the Global Strategy for Plant Conservation Conserving Europe’s threatened plants Progress towards Target 8 of the Global Strategy for Plant Conservation By Suzanne Sharrock and Meirion Jones May 2009 Recommended citation: Sharrock, S. and Jones, M., 2009. Conserving Europe’s threatened plants: Progress towards Target 8 of the Global Strategy for Plant Conservation Botanic Gardens Conservation International, Richmond, UK ISBN 978-1-905164-30-1 Published by Botanic Gardens Conservation International Descanso House, 199 Kew Road, Richmond, Surrey, TW9 3BW, UK Design: John Morgan, [email protected] Acknowledgements The work of establishing a consolidated list of threatened Photo credits European plants was first initiated by Hugh Synge who developed the original database on which this report is based. All images are credited to BGCI with the exceptions of: We are most grateful to Hugh for providing this database to page 5, Nikos Krigas; page 8. Christophe Libert; page 10, BGCI and advising on further development of the list. The Pawel Kos; page 12 (upper), Nikos Krigas; page 14: James exacting task of inputting data from national Red Lists was Hitchmough; page 16 (lower), Jože Bavcon; page 17 (upper), carried out by Chris Cockel and without his dedicated work, the Nkos Krigas; page 20 (upper), Anca Sarbu; page 21, Nikos list would not have been completed. Thank you for your efforts Krigas; page 22 (upper) Simon Williams; page 22 (lower), RBG Chris. We are grateful to all the members of the European Kew; page 23 (upper), Jo Packet; page 23 (lower), Sandrine Botanic Gardens Consortium and other colleagues from Europe Godefroid; page 24 (upper) Jože Bavcon; page 24 (lower), Frank who provided essential advice, guidance and supplementary Scumacher; page 25 (upper) Michael Burkart; page 25, (lower) information on the species included in the database. -

Floral Biology, Microclimate, and Pollination by Ectothermic Bees in an Early-Blooming Herb1
Ecology, 76(1), 1995, pp. 218-228 0 1995 by the Ecological Society of America FLORAL BIOLOGY, MICROCLIMATE, AND POLLINATION BY ECTOTHERMIC BEES IN AN EARLY-BLOOMING HERB1 CARLOSM. HERRERA Estacidn Bioldgica de Doriana, CSIC, Apartado 1056, E-41080 Sevilla, Spain Abstract. Abiotic factors may constrain the functioning of species interactions such as plant-pollinator mutualisms. I investigated how thermal environment affects the inter- action between the early-blooming daffodil, Narcissus longispathus (Amaryllidaceae) and its major bee pollinator (Andrena bicolor; Andrenidae), focusing simultaneously on plant and pollinator sides of the interaction. I studied fruit and seed set, flower duration, and the intrafloral thermal environment of N. longispathus, and the thermal biology, foraging be- havior, and thermoregulatory ability of A. bicolor, over a 6-yr period in southeastern Spain. N. longispathus flowers from February to April, when unsuitable weather often limits pollinator activity, yet most flowers are successfully pollinated in all years and sites. Fruit set was weakly pollen limited, but among flowers setting fruit the proportion of ovules developing into seeds was not. Individual flowers lasted for 17 d on average, remaining functional during this period. On sunny days, the air inside N. longispathus flowers was significantly warmer than outside. Mean temperature excess inside flowers was as high as 8"C, and was positively related to solar irradiance. Within flowers, air temperature was highest around the anthers; this intrafloral gradient was consistent with variation among perianth parts in radiation transmittance. Andrena bicolor foraged in N. longispathus flowering patches only on sunny days with air temperature >12"-13"C, and foraging behavior and flower visitation rate were temper- ature dependent. -

A Common Threat to IUCN Red-Listed Vascular Plants in Europe
Tourism and recreation: a common threat to IUCN red-listed vascular plants in Europe Author Ballantyne, Mark, Pickering, Catherine Marina Published 2013 Journal Title Biodiversity and Conservation DOI https://doi.org/10.1007/s10531-013-0569-2 Copyright Statement © 2013 Springer. This is an electronic version of an article published in Biodiversity and Conservation, December 2013, Volume 22, Issue 13-14, pp 3027-3044. Biodiversity and Conservation is available online at: http://link.springer.com/ with the open URL of your article. Downloaded from http://hdl.handle.net/10072/55792 Griffith Research Online https://research-repository.griffith.edu.au Manuscript 1 Tourism and recreation: a common threat to IUCN red-listed vascular 1 2 3 4 2 plants in Europe 5 6 7 8 3 *Mark Ballantyne and Catherine Marina Pickering 9 10 11 12 4 Environmental Futures Centre, School of Environment, Griffith University, Gold Coast, 13 14 5 Queensland 4222, Australia 15 16 17 18 6 *Corresponding author email: [email protected], telephone: +61(0)405783604 19 20 21 7 22 23 8 24 25 9 26 27 28 10 29 30 11 31 32 12 33 34 13 35 36 37 14 38 39 15 40 41 16 42 43 17 44 45 46 18 47 48 19 49 50 20 51 52 21 53 54 55 22 56 57 23 58 59 24 60 61 62 63 64 65 25 Abstract 1 2 3 4 26 Tourism and recreation are large industries employing millions of people and contribute over 5 6 27 US$2.01 trillion to the global economy. -

Flowering Phenology and Reproductive Biology in Subtropical Geophytes: Case Studies with Sympatric Species of Amaryllidaceae
UNIVERSIDADE ESTADUAL DE CAMPINAS INSTITUTO DE BIOLOGIA NATHÁLIA SUSIN STREHER FLOWERING PHENOLOGY AND REPRODUCTIVE BIOLOGY IN SUBTROPICAL GEOPHYTES: CASE STUDIES WITH SYMPATRIC SPECIES OF AMARYLLIDACEAE FENOLOGIA DA FLORAÇÃO E BIOLOGIA REPRODUTIVA EM GEÓFITAS SUBTROPICAIS: ESTUDOS DE CASO COM ESPÉCIES SIMPÁTRICAS DE AMARYLLIDACEAE CAMPINAS 2016 NATHÁLIA SUSIN STREHER FLOWERING PHENOLOGY AND REPRODUCTIVE BIOLOGY IN SUBTROPICAL GEOPHYTES: CASE STUDIES WITH SYMPATRIC SPECIES OF AMARYLLIDACEAE FENOLOGIA DA FLORAÇÃO E BIOLOGIA REPRODUTIVA EM GEÓFITAS SUBTROPICAIS: ESTUDOS DE CASO COM ESPÉCIES SIMPÁTRICAS DE AMARYLLIDACEAE Dissertation presented to the Institute of Biology of the University of Campinas in partial fulfillment of the requirements for the degree of Master in the area of Plant Biology Dissertação apresentada ao Instituto de Biologia da Universidade Estadual de Campinas como parte dos requisitos exigidos para a obtenção do Título de Mestra em Biologia Vegetal. ORIENTADOR: JOÃO SEMIR COORIENTADORA: JULIE HENRIETTE ANTOINETTE DUTILH ESTE ARQUIVO DIGITAL CORRESPONDE À VERSÃO FINAL DA DISSERTAÇÃO DEFENDIDA PELA ALUNA NATHÁLIA SUSIN STREHER E ORIENTADA PELO PROF. DR. JOÃO SEMIR. CAMPINAS 2016 Campinas, 22 de fevereiro de 2016. COMISSÃO EXAMINADORA Prof. Dr. João Semir Prof. Dr. Vinícius Lourenço Garcia de Brito Profa. Dra. Marlies Sazima Profa. Dra. Kayna Agostini Profa. Dra. Marina Wolowski Torres Os membros da Comissão Examinadora acima assinaram a Ata de defesa, que se encontra no processo de vida acadêmica do aluno. AGRADECIMENTOS Agradeço aos meus orientadores, João e Julie, por terem me dado a oportunidade de chegar neste ponto. Por terem se dedicado a mim não só profissionalmente, mas pessoalmente também. Agradeço por cada contribuição de vocês para a botânica, espero um dia saber um pouquinho do que vocês sabem. -
Monocot Nursery Seed List 1995-96
Seed List 1995-96 MONOCOT NURSERY `Jacklands', Jacklands Bridge, Tickenham, Clevedon, Avon BS21 6SG England. Only the Narcissus pages are included here. All the seeds offered in this list have been harvested this year from plants growing in the nursery or from plants in their native habitat. Reference is recommended to:- The Bulb Book' by Rix & Phillips, 'Dwarf Bulbs' and 'Larger bulbs' both by Brian Mathew or 'Bulbs' by Grey-Wilson & Mathew for descriptions and/or illustrations of most of the plants listed here. All species are prefixed by a letter and number. Please order by the letter and number only. Those prefixed 'A" are .50p per packet ` B' are 1.00p per packet ' C' are 1.50p per packet `DI are 2.00p per packet The difference in price is not an indication of quantity or rarity, either in the wild or in cultivation but the ease with which good seed is obtained. The number of seeds in each packet is not given but is in my opin- ion sufficient to sow a four inch pot to allow for two years growth before potting on Though good stocks of most of the species listed are available inevitably some will be in greater demand than others and consequently will be exhausted before all orders are filled. It will be a considerable help to me if custom- ers include a few alternatives on the order form, particularly if several 'CI or 130 items are requested. This will minimise the work involved in refunds and therefore enable me to keep prices low. -

UNZ\Rersm of TORONTO
PHYLOC;ENETIC ANALY SIS OF BREEDING-SYSTEM EVOLUTION IN EETEFtOSTYLOUS MONOCOTYLEDONS SEAN W. GRAHAM A THESIS SUBMTED IN CONFORMITY WlTH REQUTREMENTS FOR THE DEGREE OF DOCTOR OF PHILOSOPHY IN THE UNZ\rERSm OF TORONTO National Library Bibliothëque nationale I*m of Canada du Canada Acquisitions and Acquisitions et Bibliographie Services services bibliographiques 395 Wellington Street 395, me Wellington Ottawa ON K 1A ON4 OttawaON K1AON4 Canada Canada Your hie Votre relenmce our nie Naire reterence The author has granted a non- L'auteur a accordé une licence non exclusive licence allowing the exclusive permettant à la National Library of Canada to Bibliothèque nationale du Canada de reproduce, loan, distribute or sel1 reproduire, prêter, distribuer ou copies of this thesis in microfonn, vendre des copies de cette thèse sous paper or electronic formats. la forme de microfiche/nlm, de reproduction sur papier ou sur format électronique. The author retains ownership of the L'auteur conserve la propriété du copyright in this thesis. Neither the droit d'auteur qui protège cette thèse. thesis nor substantid extracts from it Ni la thèse ni des extraits substantiels may be printed or otherwise de celle-ci ne doivent être imprimés reproduced without the author's ou autrement reproduits sans son permission. autorisation. Canada PI-IYLOGE~JE~ICANALYSIS OF BREEDING-SYSTEMEVOL~ON IN HE?EROS?rtOUS MONOCOTYLEDONS Sean W. Graham, Department of Botany, University of Toronto The evolutionary histories of various stylar conditions (heierostyly, enantiostyly, stylar dirnorphism and monomorphism) were examined in two groups of rnonocotyledons, Pontederiaceae and Narcissus, using several soures of phylogenetic information and severai different weighting schemes for reconstructions of c haracter evolution. -

Multi-Scale Genetics Analyses of Two Trillium Species (Trillium
MULTI-SCALE GENETICS ANALYSES OF TWO TRILLIUM SPECIES (TRILLIUM RELIQUUM AND TRILLIUM CUNEATUM) IN SPACE AND TIME by EVA B. GONZALES (Under the Direction of J. L. HAMRICK) ABSTRACT This dissertation was motivated by an interest to integrate spatial-temporal considerations into understanding the evolutionary history of two forest herbaceous species, Trillium reliquum and T. cuneatum, and those mechanisms governing their population genetic processes. The investigation of T. reliquum addressed a hypothesis that this endangered species, surviving today in relict, disjunct populations, was previously widespread, and that it became rare due to European settlements and subsequent habitat fragmentation. Comparisons of the distribution of genetic diversity among populations of T. reliquum and T. cuneatum, its more common. albeit also fragmented, congener, revealed strong genetic structure among populations of both species. However, the disjunct T. reliquum populations are much more divergent than those of T. cuneatum, in spite of their shared recent history, suggesting that rarity in T. reliquum is more ancient, possibly predating the last glacial episode, rather than a consequence of post- European colonization. Examination of hypotheses emerging from biogeographical and fossil records regarding glacial refugia of T. cuneatum in the southeastern US revealed multiple refugia. Surprisingly, the Lower Mississippi Valley refugium, considered by paleoecologists as the main refuge for deciduous forest species, did not participate in postglacial expansion. Rather, scattered refugial populations in Alabama, Georgia and the southern Appalachian Mountains contributed to the current geographic distribution. Even more unexpected is the conclusion that T. cuneatum must have survived at more northern latitudes than the fossil record indicates. Furthermore, this study identified the Ridge and Valley as a corridor for species migration in their response to post- glacial climatic changes. -

(Asparagales: Amaryllidaceae): Especies Endémicas De España
20210624 Notas sobre el género Narcissus Linnaeus, 1753 (Asparagales: Amaryllidaceae): Especies endémicas de España PEDRO GÓMEZ-MURILLO Independent Researcher. 29680-Estepona, Málaga, Spain. [email protected] Resumen: GÓMEZ-MURILLO (2021). Notas sobre el género Narcissus Linnaeus, 1753 (Asparagales: Amaryllidaceae): Especies endémicas de España. España es uno de los países más ricos en narcisos silvestres, el objetivo de la presente investigación es establecer el inventario de narcisos silvestres endémicos de España. Se realizaron salidas de campo y una revisión de literatura especializada. Se registraron 23 especies endémicas, de las cuales solo 5 se encuentran evaluadas por la IUCN. Palabras clave: Conservación, España, Distribución, Clasificación, Diversidad. Abstract: GÓMEZ-MURILLO (2021). Notes on the genus Narcissus Linnaeus, 1753 (Asparagales: Amaryllidaceae): Endemic species of Spain. Spain is one of the richest countries in wild daffodils, the objective of this research is to establish the inventory of wild daffodils endemic to Spain. Field trips and a review of specialized literature were carried out. 23 endemic species were registered, of which only 5 are evaluated by the IUCN. Keywords: Conservation, Spain, Distribution, Classification, Diversity. OBSERVACIONES En España podemos encontrar alrededor de 60 especies del género Narcissus Linnaeus, 1753, siendo el país con más especies en estado silvestre del mundo (Aedo, 2013; Marques et al., 2017). En los últimos años se han descrito especies nuevas en el país (Escobar, -

A Three-Genome Five-Gene Comprehensive Phylogeny of The
1 A three-genome five-gene comprehensive phylogeny of the 2 bulbous genus Narcissus (Amaryllidaceae) challenges current 3 classifications and reveals multiple hybridization events 4 5 Isabel Marques1,2*, Javier Fuertes Aguilar3, Maria Amélia Martins-Louçao4, Farideh 5 3 6 Moharrek , Gonzalo Nieto Feliner 7 1Department of Agricultural and Environmental Sciences, High Polytechnic School of 8 Huesca, University of Zaragoza, Carretera Cuarte km 1, 22071 Huesca, Spain. 9 2UBC Botanical Garden & Centre for Plant Research and Department of Botany, 10 University of British Columbia, 3529-6270 University Blvd, Vancouver BC V6T 1Z4, 11 Canada. 12 3Real Jardín Botánico, CSIC, Plaza de Murillo 2, Madrid 28014, Spain. 13 4Centre for Ecology, Evolution and Environmental Changes. Faculdade de Ciências. 14 University of Lisbon. Lisbon, Portugal. 15 5Department of Plant Biology, Faculty of Biological Sciences, Tarbiat Modares 16 University, Tehran 14115-154, Iran. 17 *Corresponding author: Isabel Marques ([email protected]) 18 19 Running title: A three-genome phylogeny of Narcissus 20 1 21 Abstract 22 Besides being one of the most popular ornamental bulbs in western horticulture, the 23 Mediterranean genus Narcissus has been the subject of numerous studies focusing on a 24 wide scope of topics, including cytogenetics, hybridization and the evolution of 25 polymorphic sexual systems. Phylogenetic hypotheses based on chloroplast data have 26 provided a backbone for the genus but a detailed phylogenetic framework is still 27 lacking. To fill this gap, we present a phylogenetic study of the genus using five 28 markers from three genomes: ndhF and matK (chloroplast DNA), cob and atpA 29 (mitochondrial DNA), and ITS (nuclear ribosomal DNA). -

A List of Daffodils
TRESWITIIIAN DAFFODIL FARM Camborne Cornwall tb. Printed by . A LIST The Cam borne Printing and Stationery Co., Ltd. 24 Commercial Street Camborne, Cornwall Telephone - - 2170 OF DAFFODILS Grown by Alec Gray 1960 ALEC GRAY Treswithian Daffodil Farm, Camborne, Cornwall Phone : CAMBORNE 2041 FOREWORD Those familiar with my list in past years will notice that I have included in it this season some line illustrations ; these will, I hope, enable those who are unfamiliar with the dwarf daffodils, and who are unable to visit shows, to form some idea of what the members of the various groups are like. Customers often ask me, when ordering bulbs, for some information as to how they should be grown. To avoid having to reply to each letter individually, I am also including brief cultural instructions which, I trust, will answer most of the questions asked. Where a variety is unpriced in my list, it indicates that my stock is too small to allow me to offer it for general sale. In some instances I have less than half a dozen bulbs, but in others I have enough to be able to spare a bulb or two to anyone especially interested. Please place your orders early, as I have generally sold out of all but a few sorts by the end of June, and I replant all unsold bulbs during September. I always welcome letters from other devotees of the miniature daffodil, and am very pleased to buy or exchange bulbs of the newer, or scarcer sorts. I can still supply a reprint of my lecture to the R.H.S. -
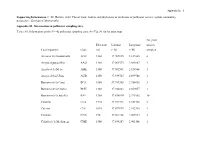
Supporting Information. C. M. Herrera. 2020. Flower Traits, Habitat, and Phylogeny As Predictors of Pollinator Service: a Plant Community Perspective
Appendix S1 – 1 Supporting Information. C. M. Herrera. 2020. Flower traits, habitat, and phylogeny as predictors of pollinator service: a plant community perspective. Ecological Monographs. Appendix S1. Information on pollinator sampling sites. TABLE S1. Information on the N = 42 pollinator sampling sites. See Fig. S1 for location map. No. plant Elevation Latitude Longitude species Local toponym Code (m) (º N) (º W) sampled Arenales del Guadalentín AGU 1360 37.909835 2.837405 4 Arroyo Aguaderillos AAG 1180 37.961573 2.883887 1 Arroyo de la Mesa AME 1100 37.902591 2.928966 1 Arroyo de los Ubios AUB 1250 37.939725 2.899766 1 Barranco de la Canal BCA 1580 37.789385 2.956438 3 Barranco de la Charca BCH 1360 37.942843 2.859057 1 Barranco de la Juanfría BJU 1380 37.836814 2.975302 10 Calarilla CLA 1710 37.944316 2.851708 2 Calerón CLE 1075 37.897973 2.942710 1 Cantalar CAN 770 37.964158 2.907974 3 Cañada de la Medianega CME 1580 37.894287 2.903146 1 Appendix S1 – 2 Cañada Pajarera CPA 1640 37.927456 2.805615 1 Collado del Calvario CCA 1420 37.950202 2.885484 1 Collado Zamora CZA 1420 37.839329 2.999615 2 Coto del Valle CVA 850 37.930542 2.928517 7 Cuesta del Bazar CBA 1330 37.906523 2.907470 1 Cuevas Bermejas CBE 1160 37.966959 2.851117 5 Fuente Bermejo FBE 1550 37.932268 2.840672 14 Fuente la Reina FRE 1460 37.941698 2.833632 2 Guadalentín GUA 1310 37.900404 2.836944 8 Hoyos de Muñoz HMU 1110 37.984831 2.876597 3 La Cabrilla LCA 1600 37.932364 2.780637 5 La Fresnedilla LFR 1080 37.980387 2.855875 2 La Mesa LME 1610 37.889823 2.913788 2 Las -

Conservation of Natural Habitats and of Wild Fauna and Flora
22 . 7 . 92 Official Journal of the European Communities No L 206 / 7 \ II (Acts whose publication is not obligatory) COUNCIL COUNCIL DIRECTIVE 92/43 / EEC of 21 May 1992 on the conservation of natural habitats and of wild fauna and flora THE COUNCIL OF THE EUROPEAN COMMUNITIES , Whereas , in the European territory of the Member States , natural habitats are continuing to deteriorate and an increasing number of wild species are seriously threatened ; Having regard to the Treaty establishing the European whereas given that the threatened habitats and species form Economic Community, and in particular Article 130s part of the Community's natural heritage and the threats to thereof, them are often of a transboundary nature , it is necessary to take measures at Community level in order to conserve Having regard to the proposal from the Commission ('), them ; Having regard to the opinion of the European Whereas , in view of the threats to certain types of natural Parliament ( 2 ), habitat and certain species , it is necessary to define them as having priority in order to favour the early implementation of Having regard to the opinion of the Economic and Social measures to conserve them ; Committee ( 3 ), Whereas , in order to ensure the restoration or maintenance Whereas the preservation , protection and improvement of of natural habitats and species of Community interest at a the quality of the environment , including the conservation of favourable conservation status, it is necessary to designate natural habitats and of wild fauna and flora