Anti-Proliferative Effect of Allium Senescens L. Extract in Human T-Cell Acute Lymphocytic Leukemia Cells
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Karyologická Variabilita Vybraných Taxonů Rodu Allium V Evropě Alena
UNIVERZITA PALACKÉHO V OLOMOUCI Přírodov ědecká fakulta Katedra botaniky Karyologická variabilita vybraných taxon ů rodu Allium v Evrop ě Diplomová práce Alena VÁ ŇOVÁ obor: T ělesná výchova - Biologie Prezen ční studium Vedoucí práce: RNDr. Martin Duchoslav, Ph.D. Olomouc 2011 Prohlašuji, že jsem zadanou diplomovou práci vypracovala samostatn ě s použitím citované literatury a konzultací. V Olomouci dne: 14.1.2011 ................................................. Pod ěkování Ráda bych pod ěkovala všem, co mi v jakémkoli ohledu pomohli. P ředevším svému vedoucímu diplomové práce RNDr. Martinu Duchoslavovi, PhD., a to nejen za cenné rady a pomoc p ři práci, ale p ředevším za velké množství trp ělivosti. Stejn ě tak d ěkuji Mgr. Míše Jandové za veškerý čas, který mi v ěnovala, Tereze P ěnkavové za pomoc ve skleníku a odd ělení fytopatologie za možnost využívat jejich laborato ří. Samoz řejm ě mé díky pat ří i všem blízkým, kte ří m ě po dobu studia podporovali. Bibliografická identifikace Jméno a p říjmení autora : Alena Vá ňová Název práce : Karyologická variabilita vybraných taxon ů rodu Allium v Evrop ě. Typ práce : Diplomová Pracovišt ě: Katedra botaniky, P řírodov ědecká fakulta Univerzity Palackého v Olomouci Vedoucí práce : RNDr. Martin Duchoslav, Ph.D. Rok obhajoby práce : 2011 Abstrakt : Diplomová práce m ěla za cíl postihnout karyologickou variabilitu (chromozomový po čet, ploidní úrove ň a DNA-ploidní úrove ň) a velikost jaderné DNA (2C) vybraných taxon ů rodu Allium pro populace získané z různých částí Evropy. Celkov ě bylo pomocí karyologických metod prov ěř eno 550 jedinc ů u 14 taxon ů rodu Allium : A. albidum, A. -

Lurvey Garden Center Blue Corkscrew Onion
2550 E. Dempster St. 1819 N. Wilke Road 496 Old Skokie Hwy. 30560 N. Russell Rd. Des Plaines, IL Arlington Heights, IL Park City, IL Volo, IL 847-824-7411 847-255-5800 847-249-7670 815-363-4420 www.lurvey.com Blue Corkscrew Onion Allium senescens 'var. glauca' Plant Height: 12 inches Flower Height: 24 inches Spread: 12 inches Sunlight: Hardiness Zone: 2 Other Names: Flowering Onion Description: Spiraling blue-green foliage and lavender flowers create a beautiful display in garden beds, borders or containers; foliage last throughout most of the summer months, while Blue Corkscrew Onion flowers flowers generally bloom in late spring; deadhead after Photo courtesy of NetPS Plant Finder flowering to stop seeding Ornamental Features Blue Corkscrew Onion has masses of beautiful balls of lightly-scented lavender flowers at the ends of the stems from late spring to early summer, which are most effective when planted in groupings. Its sword-like leaves remain bluish-green in color throughout the season. The fruit is not ornamentally significant. Landscape Attributes Blue Corkscrew Onion is an open herbaceous perennial with tall flower stalks held atop a low mound of foliage. Its Blue Corkscrew Onion flowers medium texture blends into the garden, but can always Photo courtesy of NetPS Plant Finder be balanced by a couple of finer or coarser plants for an effective composition. This is a relatively low maintenance plant, and should only be pruned after flowering to avoid removing any of the current season's flowers. It is a good choice for attracting butterflies to your yard, but is not particularly attractive to deer who tend to leave it alone in favor of tastier treats. -

Garlic, an Approach to Healthy Life
Sharma N et al / IJRAP 2010, 1 (2) 358-366 Review Article Available online through www.ijrap.net NATURAL HEALING AGENT: GARLIC, AN APPROACH TO HEALTHY LIFE Nagori B.P., Solanki Renu, Sharma Neha* Lachoo Memorial College of Science and Technology, Pharmacy Wing, Jodhpur, India Received: 03-11-2010; Revised: 28-11-2010; Accepted: 03-12-2010 ABSTRACT We have grown up in the era of so-called wonder drugs. Garlic is one such drug which is grown globally. China is by far the largest producer of garlic, with approximately 10.5 million tonnes (23 billion pounds) annually, accounting for over 77% of world output. This leaves 16% of global garlic production in countries that each produces less than 2% of global output. The purpose of this study is to highlight new applications of cultivated as well as wild garlic in medicine. Areas of beneficial activity include anti- AIDS, anti-cancer and anti-cardiovascular disease and anti-infectious properties, amongst others. Garlic is uniquely the richest dietary source of many otherwise rare healthful sulphur compounds, plus organic selenium and germanium besides other essential nutrients and active health-promoting phytochemicals. Various forms of garlic are available, the most effective being fresh, powdered, distilled and especially aged garlic, which later lacks the irritant effect of fresh garlic, yet possesses equal or greater bio-active range and potency. Since many years cultivated garlic (Allium sativum) has served the medicinal purpose. As demand of garlic is continuously increasing due to its valuable features, other garlic species are screened for potential benefits of cultivated garlic with less side effects. -

Red List of Vascular Plants of the Czech Republic: 3Rd Edition
Preslia 84: 631–645, 2012 631 Red List of vascular plants of the Czech Republic: 3rd edition Červený seznam cévnatých rostlin České republiky: třetí vydání Dedicated to the centenary of the Czech Botanical Society (1912–2012) VítGrulich Department of Botany and Zoology, Masaryk University, Kotlářská 2, CZ-611 37 Brno, Czech Republic, e-mail: [email protected] Grulich V. (2012): Red List of vascular plants of the Czech Republic: 3rd edition. – Preslia 84: 631–645. The knowledge of the flora of the Czech Republic has substantially improved since the second ver- sion of the national Red List was published, mainly due to large-scale field recording during the last decade and the resulting large national databases. In this paper, an updated Red List is presented and compared with the previous editions of 1979 and 2000. The complete updated Red List consists of 1720 taxa (listed in Electronic Appendix 1), accounting for more then a half (59.2%) of the native flora of the Czech Republic. Of the Red-Listed taxa, 156 (9.1% of the total number on the list) are in the A categories, which include taxa that have vanished from the flora or are not known to occur at present, 471 (27.4%) are classified as critically threatened, 357 (20.8%) as threatened and 356 (20.7%) as endangered. From 1979 to 2000 to 2012, there has been an increase in the total number of taxa included in the Red List (from 1190 to 1627 to 1720) and in most categories, mainly for the following reasons: (i) The continuing human pressure on many natural and semi-natural habitats is reflected in the increased vulnerability or level of threat to many vascular plants; some vulnerable species therefore became endangered, those endangered critically threatened, while species until recently not classified may be included in the Red List as vulnerable or even endangered. -

Green Factor Plant List 2010
Revised December 2010 Seattle Green Factor Plant List Notes: ● All plants on this list are drought-tolerant once they are established unless comments indicate otherwise. ● Seattle Department of Transportations Right-of Way Improvement Manual establishes height limits for non-street-tree plantings in rights-of-way. Maximum plant height within 30 feet of an intersection (as measured from the corner of the curb) is 24 inches. Elsewhere in the right-of-way, plantings are allowed to be 30 inches tall. ● "Bioretention Zone" describes where plants can appropriately be used in bioretention systems such as swales and rain gardens. Zone 1 is the designation for plants that can be used in the flat bottoms of bioretention facilities: 1A refers to species that prefer soil saturation or shallow inundation for long durations, while Zone 1B refers to plants that can alternate between dry ands short-term saturated conditions. Zone 2 is the designation for plants best used at the well-drained slopes of bioretention facilities. All other species are appropriate for planting at the tops of bioretention areas. GROUNDCOVERS Scientific Name Common Name Evergreen Shade Sun Native up to 24" 2-3' ht Bioretention Zone Notes Arctostaphylos uva-ursi kinnikinnick ●●●● Asarum caudatum wild ginger ●● ● Calluna , in variety heather ●●● Ceratostigma plumbaginoides hardy plumbago ●●● ● Daboecia cantabrica Irish heath ●●● Erica , in variety heath ●●● Erigeron karvinskianus Latin American fleabane ●●● ● Euonymous fortunei 'Colorata' wintercreeper euonymous ●●● ● Festuca -

Comparative Susceptibility of Onion Varieties and of Species of Allium to Urocystis Cepulae1
COMPARATIVE SUSCEPTIBILITY OF ONION VARIETIES AND OF SPECIES OF ALLIUM TO UROCYSTIS CEPULAE1 By P. J. ANDERSON Formerly Research Professor of Botany, Massachusetts Agricultural Experiment Station INTRODUCTION Most pathogenic fungi show a distinct variation in their ability to infect different varieties of their host plant. Such a difference in varietal susceptibility is usually the starting point for the breeding or selection of resistant varieties, which is the most effectual method for the control of plant diseases. With respect to onion smut, how- ever, it has never been shown that there is any difference in the sus- ceptibility of the numerous varieties under cultivation; in fact, no very comprehensive variety tests with this objective in view have been conducted. Walker and Jones {12y tested a few varieties, and Whitehead (13, p. 44-9) in England tested 21 varieties of onions and 11 varieties of leeks (Allium porrum), but none showed resistance. Nevertheless, since there were numerous other varieties which had not been tested, so far as was known to the writer, it appeared worth while to collect seed of as many varieties as could be obtained and test them comparatively in the hope that some of them might show a degree of resistance which should warrant crossing and selection work. The genus Allium, to which the cultivated onion belongs, is a large genus of some 250 species which are widely distributed over the earth. Urocystis cepulae was first reported on Allium cepa (4, 8) in 1857, in eastern Massachusetts. As early as 1881 (7) it was also reported on A. porrum (leek), in France. -

Karyotypes of Allium Senescens L. Ssp. Montanum (Fries) Holub Populations from the Mt
Acta Bot. Croat. 60 (2), 177-186,2001 CODEN: ABCRA25 ISSN 0365-0588 UDC 575.822:582.57 Dedicated to Prof. dr. sc. ZVONIMIR DÉVIDÉ on the occasion of his 80th birthday Karyotypes of Allium senescens L. ssp. montanum (Fries) Holub populations from the Mt. Biokovo region V išnja B esendorfer1*, Klaudija Šop1, Marija-Edita Šolić2, D ražena Papeš1 1 Department of Molecular Biology, Faculty of Science, University of Zagreb, Rooseveltov trg 6/III, HR-10000 Zagreb, Croatia 2 Mountain and the Sea Institute, Makarska, Croatia The karyotypes of three Allium senescens ssp. montanum (Fries) Holub populations from the Mt Biokovo region were investigated. This species has been poorly cytogenetically analysed, and with respect to Croatian populations such investigations have never been carried out. The chromosome number, morphology and distribution of heterochromatin were investigated. The number, position and activity of nucleolar organising regions (NORs) were also analysed. In all three populations, only plants with a tetraploid (2n = 4x = 32) chromosome complement were found. The chromosome complement consisted of seven groups of four metacentric and one group of four submetacentric chromosomes bearing the satellite on the short arm. Chromosomes exhibit four types of Giemsa C-bands: telomeric, intercalary, centromeric bands and those located on satellites. Eight chromosomes with the characteristic banding pattern were distinguished. Silver staining revealed a maximum of four nucleoli that corresponded to the maximum number of active NORs (Ag-NORs) located terminally on the short arm of four submetacentric chromo somes. The results obtained suggest that A. senescens ssp. montanum from the Mt Biokovo region is an autotetraploid species. -

To View Our Perennials Plant List
Perennials! # # # # #! ! Acanthus hungaricus zone 6-9 sun A hardy Bear's breeches with bold dark green thistle like foliage and stately spires of bicolored lavender and white flowers on 3' stalks. Blooms late June-August. Gal. $15.00 ! ! Acanthus spinosus zone 6-9 sun/pt.shade The hardiest of the Bear' s Breaches, this bold accent plant has large shiny green thistle like foliage and 3' flower spikes with soft mauve blossoms. We have found that sitting it in a well- drained protected spot and a winter mulch encourages blooming. gallon $14.0 # # # # # #! Achillea ptarmica 'Angel's Breath' zone 3-8 sun GREEK YARROW. Sprays of pure double-white flowers, resembling large baby's breath, blooms in late spring are produced over a long period. Finely toothed foliage is dark green Qt. $10.00 ! ! Adiantum pedatum zone 3-8 pt.sh/shade Northern Maidenhair is one of the most admired ferns, bearing delicate bright green fronds that quiver on shiny black stems. Prefers a humus-rich neutral-acid soil. 15-18". Qt. $14.00 ! # # # # # #! ! Agastache 'Black Adder' zone 6 -9 sun A new selection of Anise Hyssop with smoky lilac flowers beginning in July and continuing to frost. This is a plant for poor well drained soil, as it gets too lanky if overfertilized and dislikes soggy roots. 2-3'. Qt. $10.00! Alchemilla mollis 'Auslese' zone 4-9 sun/pt.shade # # # # # #! A favorite edging plant for perennial and mixed borders, with lovely gray-green scalloped foliage and airy sprays of chartreuse flowers that seem to compliment every color scheme in June and July. -

Glasnik Hbod-3-1-2015
Glasnik Hrvatskog botaničkog društva 3(1) 2015. Glasnik Hrvatskog botaničkog društva Journal of the Croatian Botanical Society Allium lusitanicum Lam. (foto M. Milović) Ožujak / March 2015. Vol. 3 br. 1 Glasnik Hrvatskog botaničkog društva 3(1) 2015. Izdavač : Hrvatsko botaničko društvo ( www.hbod.hr ) Urednici : Toni Nikolić, Sandro Bogdanović Tehnički urednici: Igor Boršić, Toni Nikolić Urednički odbor: A. Alegro, N. Jasprica, Z. Liber, M. Milović, A. Plenković-Moraj, B. Pevalek-Kozlina, Ž. Škvorc Adresa uredništva: Glasnik Hrvatskog botaničkog društva, Marulićev trg 9a, HR-10000 Zagreb Dizajn, prijelom, web: T. Nikolić ISSN: 1848-8102 Web adresa: http://hirc.botanic.hr/Glasnik-HBoD/ Skraćeni naslov: Glas. Hrvat. bot. druš. Izlazi povremeno. Namjena : Glasnik Hrvatskog botaničkog društva elektronički je časopis HBoD-a namijenjen objavljivanju stručnih i znanstvenih priloga o flori i vegetaciji, te drugih podataka i informacija domaćoj, ali i inozemnoj botaničkoj i drugoj zajednici. Geografski je primarno usmjeren na područje Republike Hrvatske. Stručno i znanstveno je primarno usmjeren na objavljivanje priloga o poznavanju flore i vegetacije, nomenklaturnim pitanjima i taksonomiji, ali i drugih i raznorodnih podataka vezanih uz poznavanje nacionalne flore. Prilozi se objavljuju na hrvatskom ili engleskom jeziku. Upute autorima Mole se autori da svoje radove, rukopise, priloge, slike i tablice dostave isključivo u elektroničkom obliku na e-mail adresu urednika: [email protected] ili [email protected] . Tekst svih rukopisa treba biti napisan u MS-Wordu, font Arial, veličine slova 10, bez fusnota, s jednostrukim proredom na A4 formatu (210 x 297 mm), a stranice trebaju biti označene u donjem desnom kutu. Izvorni znanstveni radovi i stručni prilozi mogu biti napisani na hrvatskom ili engleskom jeziku. -
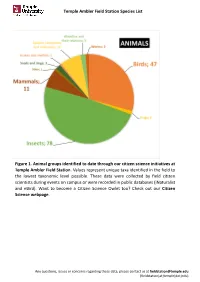
Temple Ambler Field Station Species List Figure 1. Animal Groups Identified to Date Through Our Citizen Science Initiatives at T
Temple Ambler Field Station Species List Figure 1. Animal groups identified to date through our citizen science initiatives at Temple Ambler Field Station. Values represent unique taxa identified in the field to the lowest taxonomic level possible. These data were collected by field citizen scientists during events on campus or were recorded in public databases (iNaturalist and eBird). Want to become a Citizen Science Owlet too? Check out our Citizen Science webpage. Any questions, issues or concerns regarding these data, please contact us at [email protected] (fieldstation[at}temple[dot]edu) Temple Ambler Field Station Species List Figure 2. Plant diversity identified to date in the natural environments and designed gardens of the Temple Ambler Field Station and Ambler Arboretum. These values represent unique taxa identified to the lowest taxonomic level possible. Highlighted are 14 of the 116 flowering plant families present that include 524 taxonomic groups. A full list can be found in our species database. Cultivated specimens in our Greenhouse were not included here. Any questions, issues or concerns regarding these data, please contact us at [email protected] (fieldstation[at}temple[dot]edu) Temple Ambler Field Station Species List database_title Temple Ambler Field Station Species List last_update 22October2020 description This database includes all species identified to their lowest taxonomic level possible in the natural environments and designed gardens on the Temple Ambler campus. These are occurrence records and each taxon is only entered once. This is an occurrence record, not an abundance record. IDs were performed by senior scientists and specialists, as well as citizen scientists visiting campus. -

Allium – Vasteplant Uien, Op Weg Naar Een Vaste Plaats in Het Sortiment Dr
Allium – Vasteplant uien, op weg naar een vaste plaats in het sortiment Dr. P.C. de Jong Het geslacht Allium is met ruim 700 of misschien wel 800 soorten een zeer groot geslacht. Het is dan ook niet zo verwonderlijk dat de kennis van de soorten van dit geslacht nog veel hiaten vertoont. Het geslacht bestaat uit bolgewassen, waarbij bij diverse soorten de bollen op een wortelstok staan. Deze worden gezien als vaste planten. Hieronder zijn er enige Allium nutans ‘Esmee’ die als tuinplant meer aandacht verdienen. (: ) De vasteplant uien zijn zeer winterhard en komen (124) en China (138). Op het Zuidelijk Halfrond onderscheiden in het geslacht Allium 15 onderge- zeer grote aantallen planten. Omdat A. nutans vrij in het voorjaar al vroeg in blad, dat tot in de zijn nog twee soorten bekend in Argentinië en slachten met in totaal 56 secties. Het aantal soor- onbekend is, evenals het verschil van deze soort herfst groen blij. Dit in contrast met de meeste Chili en één in Zuid Afrika. De meeste soorten ten hielden zij op het moment van de publicatie op met A. senescens, onderzocht hij mogelijk toch de bol-uien, waarvan het blad al verwelkt voor of zijn te vinden in berggebieden. Bij de soorten is ongeveer 780. Het geslacht was lang ingedeeld bij zeer variabele soort A. senescens. In zijn publicatie tijdens de bloei. Zowel de bloemen als het blad er een duidelijk onderscheid tussen de echte bol- de Liliaceae. Daarna in de aparte familie Alliaceae. wijst hij overigens op de moeilijkheid van de hebben sierwaarde. De bloeiperiode is vanaf len en de bollen op een gezamenlijke wortelstok. -

Nieuwe Oogst
NIEUWE OOGST CATALOGUS 2019 NIJSSEN BULBS HEEMSTEDE WWW.NIJSSENBULBS.NL Afbeelding omslag: Allium ‘Mount Everest’, zie pagina 24. Fotografie: Gert-Pieter Nijssen en vele enthousiaste kwekers, waarvoor mijn dank. Deze uitgave kwam tot stand door samenwerking tussen: Tekst: Gert-Pieter Nijssen en archief Peter C. Nijssen Lithografie/Produktie: LNO drukkerij, Zierikzee. Niets uit deze uitgave mag worden vermenigvuldigd en/of openbaar gemaakt door middel van druk, fotokopie, microfilm of op welke wijze ook, zonder voorafgaande toestemming van Nijssen Bulbs Heemstede. Lichte kleurafwijkingen van foto’s en eventuele zet- of drukfouten in de catalogus voorbehouden. Een bezoek aan ons plantencentrum in Heemstede is altijd de moeite waard. Adres: Sportparklaan 25A, 2103 VR Heemstede. Voor actuele openingstijden kijk op: www.nijssenbulbs.nl Maakt u gebruik van het openbaar vervoer: Vanaf station Heemstede-Aerdenhout, stadsbus 4 richting Heemstede. Na ongeveer zes minuten uitstappen bij halte Glipperdreef/Begraafplaats. Nog ongeveer vijf minuten lopen in zuidelijke richting, na ong. 200 meter linksaf, de Sportparklaan in. Het plantencentrum bevindt zich aan de linkerzijde, schuin tegenover het zwembad. Algemene voorwaarden Nijssen Bulbs Heemstede Deze kunt u raadplegen op www.nijssenbulbs.nl, maar sturen wij u ook, op verzoek, kosteloos toe. Prijs catalogus ¤ 10.00 voor Nederland, overige landen binnen de E.U. ¤ 15.00 Bol- en Knolgewassen Een Nieuwe Oogst Nijssen Bulbs Heemstede Bijzondere bol- en knolgewassen Sportparklaan 25a 2103 VR HEEMSTEDE Tel.: 023 - 5471056 Correspondentie-adres: Postbus 653 2100 AR HEEMSTEDE www.nijssenbulbs.nl [email protected] Catalogus 2019 Catalogus 1 Bij-vriendelijke bloembollenpakketten Het belang van biodiversiteit en planten in de tuin waarvan o.a.