Abdominal Masses: Solid Organs and Gastrointestinal
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

General Signs and Symptoms of Abdominal Diseases
General signs and symptoms of abdominal diseases Dr. Förhécz Zsolt Semmelweis University 3rd Department of Internal Medicine Faculty of Medicine, 3rd Year 2018/2019 1st Semester • For descriptive purposes, the abdomen is divided by imaginary lines crossing at the umbilicus, forming the right upper, right lower, left upper, and left lower quadrants. • Another system divides the abdomen into nine sections. Terms for three of them are commonly used: epigastric, umbilical, and hypogastric, or suprapubic Common or Concerning Symptoms • Indigestion or anorexia • Nausea, vomiting, or hematemesis • Abdominal pain • Dysphagia and/or odynophagia • Change in bowel function • Constipation or diarrhea • Jaundice “How is your appetite?” • Anorexia, nausea, vomiting in many gastrointestinal disorders; and – also in pregnancy, – diabetic ketoacidosis, – adrenal insufficiency, – hypercalcemia, – uremia, – liver disease, – emotional states, – adverse drug reactions – Induced but without nausea in anorexia/ bulimia. • Anorexia is a loss or lack of appetite. • Some patients may not actually vomit but raise esophageal or gastric contents in the absence of nausea or retching, called regurgitation. – in esophageal narrowing from stricture or cancer; also with incompetent gastroesophageal sphincter • Ask about any vomitus or regurgitated material and inspect it yourself if possible!!!! – What color is it? – What does the vomitus smell like? – How much has there been? – Ask specifically if it contains any blood and try to determine how much? • Fecal odor – in small bowel obstruction – or gastrocolic fistula • Gastric juice is clear or mucoid. Small amounts of yellowish or greenish bile are common and have no special significance. • Brownish or blackish vomitus with a “coffee- grounds” appearance suggests blood altered by gastric acid. -

An Abdominal Tuberculosis Case Mimicking an Abdominal Mass Derya Erdog˘ Ana, Yasemin Tascı¸ Yıldızb, Esin Cengiz Bodurog˘ Luc and Naciye Go¨ Nu¨L Tanırd
Case report 81 An abdominal tuberculosis case mimicking an abdominal mass Derya Erdog˘ ana, Yasemin Tascı¸ Yıldızb, Esin Cengiz Bodurog˘ luc and Naciye Go¨ nu¨l Tanırd Abdominal tuberculosis is rare in childhood. It may be Departments of aPediatric Surgery, bRadiology, cPathology and dPediatric difficult to diagnose as it mimics various disorders. We Infectious Diseases, Dr Sami Ulus Maternity and Children’s Research and Training Hospital, Altındag˘ -Ankara, Turkey present a 12-year-old child with an unusual clinical Correspondence to Derya Erdog˘ an, Dr Sami Ulus Maternity and Children’s presentation who was diagnosed with abdominal Research and Training Hospital, Babu¨r caddesi No. 34 06080, tuberculosis only perioperatively. Ann Pediatr Surg Altındag˘ -Ankara, Turkey Tel: + 90 542 257 5522; fax: + 90 312 317 0353; 9:81–83 c 2013 Annals of Pediatric Surgery. e-mail: [email protected] Annals of Pediatric Surgery 2013, 9:81–83 Received 1 June 2012 accepted 3 January 2013 Keywords: abdominal tuberculosis, child, diagnosis Introduction hyperemia around the umbilicus. A mass with undefined Tuberculosis continues to be an important healthcare borders that filled the whole abdomen was present, and problem, especially in developing countries. Abdominal the paraumblical area was tender on palpation. The tuberculosis is quite rare and can present with different posteroanterior chest and plain abdominal radiographs clinical features in children compared with adults. It can showed nonspecific findings (Fig. 1). Abdominal ultra- be difficult to diagnose as it can mimic various abdominal sonography revealed stage 1 hydronephrosis, minimal diseases. splenomegaly, a multiloculated cystic, and a fine septated mass 51 Â 15 mm in size adjacent to the anterior border of Case report the liver and multiloculated cystic fine septated masses A 12-year-old boy presented with increasing abdominal 51 Â 38 mm in size adjacent to the pancreas inferiorly. -

Pneumatosis Cystoides Intestinalis
vv Clinical Group Archives of Clinical Gastroenterology ISSN: 2455-2283 DOI CC By Monica Onorati1*, Marta Nicola1, Milena Maria Albertoni1, Isabella Case Report Miranda Maria Ricotti1, Matteo Viti2, Corrado D’urbano2 and Franca Di Pneumatosis Cystoides Intestinalis: Nuovo1 Report of a New Case of a Patient with 1Pathology Unit, ASST-Rhodense, Garbagnate Milanese, Italy 2Surgical Unit, ASST-Rhodense, Garbagnate Artropathy and Asthma Milanese, Italy Dates: Received: 09 January, 2017; Accepted: 07 March, 2017; Published: 08 March, 2017 Abstract *Corresponding author: Monica Onorati, MD, Pathology Unit, ASST-Rhodense, v.le Carlo Forla- Pneumatosis cystoides intestinalis (PCI) is an uncommon entity without the characteristics of a nini, 45, 20024, Garbagnate Milanese (MI), Italy, disease by itself and it is characterized by the presence of gas cysts within the submucosa or subserosa Tel: 02994302392; Fax: 02994302477; E-mail: of the gastrointestinal tract. Its precise etiology has not been clearly established and several hypotheses have been postulated regarding the pathogenesis. Since it was fi rst described by Du Vernoy in autopsy specimens in 1730 and subsequently named by Mayer as Cystoides intestinal pneumatosis in 1825, it has https://www.peertechz.com been reported in some studies. PCI is defi ned by physical or radiographic fi ndings and it can be divided into a primary and secondary forms. In the fi rst instance, no identifi able causal factors are detected whether secondary forms are associated with a wide spectrum of diseases, ranging from life-threatening to innocuous conditions. For this reason, PCI management can vary from urgent surgical procedure to clinical, conservative treatment. The clinical onset may be very heterogeneous and represent a challenge for the clinician. -

Expanding Abdominal Mass in a 41-Year-Old Patient with a History of Alcohol Abuse
CLINICAL CASE OF THE MONTH Expanding Abdominal Mass in a 41-Year-Old Patient with a History of Alcohol Abuse Racheed Ghanami, BS; Leila Obeid, MD; Betsy Buchert, BA; Scott Beech, MD; Yi-Zarn Wang, MD, DDS; and Fred A. Lopez, MD, FACP 41-year-old man with a history of significant al- minute, respiratory rate of 24 breaths per minute, and cohol use presented to an outside hospital with blood pressure of 118/56 mmHg. The patient appeared A complaints of nausea, vomiting, epigastric pain, acutely ill and lethargic. Though his speech was slurred, and subjective fever for 4 days. He also complained of he was oriented in all spheres. Physical exam further dizziness and weakness that began 1 day prior to pre- revealed temporal wasting, anicteric sclera, and dry sentation. The patient stated that he drank in excess of mucous membranes. The abdomen was not distended one case of beer the night prior to presentation. There but decreased bowel sounds were appreciated. No ten- was no history of recent trauma. In the past, he experi- derness of the abdomen was elicited with palpation, and enced sporadic episodes of abdominal pain which lasted there was no evidence of a palpable mass or hepatosple- for up to 2 days. The episodes were typically preceded by nomegaly. excessive drinking of alcohol. The patient did report a Laboratory values on admission were a white count 20-pound weight loss that occurred over the prior 2 years, of 14,000/µL (normal range, 4,500-11,000/µL), hemat- but denied chest pain, shortness of breath, post-prandial ocrit of 47.7% (normal range, 40-51%), platelets of abdominal pain, change in bowel habits, change in stool 392,000/µL (normal range, 130,000-400,000/µL), amy- color, urinary symptoms, or skin abnormalities. -
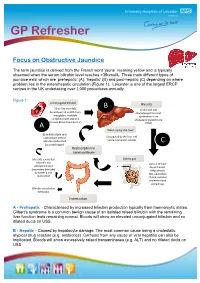
Obstructive Jaundice
GP Refresher Focus on Obstructive Jaundice The term jaundice is derived from the French word ‘jaune’ meaning yellow and is typically observed when the serum bilirubin level reaches >35umol/L. Three main different types of jaundice exist which are ‘prehepatic’ (A), ‘hepatic’ (B) and post-hepatic (C) depending on where problem lies in the enterohepatic circulation (Figure 1). Leicester is one of the largest ERCP centres in the UK undertaking over 1,000 procedures annually. Figure 1: Unconjugated bilirubin B Bile salts 70 to 90% from RBC Cholic acid and breakdown 10 to 30% from chenodeoxycholic acid, myoglobin, insoluble synthesised from complexed with albumin cholesterol (cytochrome (crosses blood-brain barrier) P450) A Taken up by the liver 80 to 90% of bile salts Conjugated by the liver and reabsorbed. 20% of bilirubin reabsorbed hence now water-soluble C (as urobilinogen) Reabsorption in terminal ileum Bile salts converted Enters gut lithocolic and Up to 1 litre per deoxycholic acid day produced. (secondary bile salts) Helps absorb by bacteria and fats, neutralises reabsorbed chyme, excretes cholesterol and some drugs Bilirubin excreted as stercobilin Enters colon A - Prehepatic - Characterised by increased bilirubin production typically from haemoloytic states. Gilbert’s syndrome is a common benign cause of an isolated raised bilirubin with the remaining liver function tests remaining normal. Bloods will show an elevated unconjugated bilirubin and no dilated ducts on USS. B - Hepatic - Caused by hepatocyte damage. The most common cause being a cholestatic atypical drug reaction (e.g. antibiotics). Cirrhosis from any cause or viral hepatitis can also be implicated. Bloods will show excessively raised transaminases (e.g. -

High Flow Oxygen Therapy for Pneumatosis Coli
Gut: first published as 10.1136/gut.20.6.493 on 1 June 1979. Downloaded from Gut, 1979, 20, 493-498 High flow oxygen therapy for pneumatosis coli S. HOLT1, H. M. GILMOUR, T. A. S. BUIST, K. MARWICK, AND R. C. HEADING From the Departments of Therapeutics and Clinical Pharmacology, Pathology, and Diagnostic Radiology, The Royal Infirmary, Edinburgh SUMMARY Symptomatic and radiological resolution of pneumatosis coli was achieved by intermit- tent high flow oxygen therapy in five patients. In each case the extent of the disease was defined by colonoscopy and contrast radiography before treatment. Despite the confirmation of pneumocyst resolution, recurrence of colonic gas cysts was noted in two patients at six months and one year after treatment. Bacteriological studies indicated that resolution of the disease, induced by oxygen therapy, was not associated with eradication of anerobic bacteria from stool and colonic mucosa. The clinical features and response to treatment ofthis group ofpatients are discussed, with particular reference to previously reported methods of oxygen administration. Primary pneumatosis coli is a disease of unknown Although a number of authors have reported aetiology, in which multiple gas-filled cysts ofvarying resolution of pneumatosis cystoides intestinalis with size and distribution are found in the large intestine. oxygen therapy, there is no published series in which Its prevalence in the general population is difficult to a consistent investigative and therapeutic approach assess as the disease may be asymptomatic, but it has been adopted. We describe five patients with seems to be rare. We have previously noted an primary pneumatosis coli, in whom symptomatic and http://gut.bmj.com/ incidence of two cases in 6553 consecutive post radiological resolution of disease has resulted from a mortem examinations (Holt et al., 1978). -

Patients with Positive Screening Fecal Occult Blood Tests
Department of Veterans Affairs Health Services Research & Development Service Evidence-based Synthesis Program Patients with Positive Screening Fecal Occult Blood Tests: Evidence Brief on the Relationship Between Time Delay to Colonoscopy and Colorectal Cancer Outcomes SUPPLEMENTARY MATERIALS April 2013 Prepared for: Investigators: Department of Veterans Affairs Principal Investigator: Veterans Health Administration Kim Peterson, MS Quality Enhancement Research Initiative Health Services Research & Development Service Contributing Investigators: Washington, DC 20420 Susan Carson, MPH Linda Humphrey, MD, MPH Prepared by: Mark Helfand, MD, MPH, MS Evidence-based Synthesis Program (ESP) Coordinating Center Portland VA Medical Center Portland, OR Mark Helfand, MD, MPH, MS, Director Colonoscopy Delay in FOBT-Positive Patients Supplemental Materials Evidence-based Synthesis Program TABLE OF CONTENTS SEARCH STRATEGY ...................................................................................................................................... 1 EXCLUDED STUDIES ..................................................................................................................................... 2 TABLE 1. DATA ABSTRACTION .............................................................................................................. 5 TABLE 2. QUALITY RATINGS ................................................................................................................. 9 REFERENCES .................................................................................................................................................. -

In the Clinic
in the clinic Irritable Bowel Syndrome Diagnosis page ITC7-2 Treatment page ITC7-8 Practice Improvement page ITC7-14 Patient Information Page page ITC7-15 CME Questions page ITC7-16 Section Editors The content of In the Clinic is drawn from the clinical information and Christine Laine, MD, MPH education resources of the American College of Physicians (ACP), including David Goldmann, MD PIER (Physicians’ Information and Education Resource) and MKSAP (Medical Knowledge and Self-Assessment Program). Annals of Internal Medicine Science Writer editors develop In the Clinic from these primary sources in collaboration with Jennifer F. Wilson the ACP’s Medical Education and Publishing Division and with the assistance of science writers and physician writers. Editorial consultants from PIER and MKSAP provide expert review of the content. Readers who are interested in these primary resources for more detail can consult http://pier.acponline.org and other resources referenced in each issue of In the Clinic. The information contained herein should never be used as a substitute for clinical judgment. © 2007 American College of Physicians Downloaded From: http://annals.org/ by McGill University, Teresa Rudkin on 04/08/2016 in the clinic rritable bowel syndrome (IBS) is a common but poorly understood dis- order that interferes with normal colon function, resulting in abdominal I pain, bloating, constipation, and diarrhea. No specific biological bio- marker, physiologic abnormality, or anatomical defect has been discovered. Psychosocial stress may exacerbate symptoms. IBS is 1 of 28 adult and 17 pediatric functional gastrointestinal disorders. These disorders are symptom-based and not explained by other pathologically defined diseases. -

A Review of Pneumatosis Intestinalis in the Setting of Systemic Cancer Treatments, Including Tyrosine Kinase Inhibitors
View metadata, citation and similar papers at core.ac.uk brought to you by CORE provided by Elsevier - Publisher Connector Canadian Association of Radiologists Journal 63 (2012) 312e317 www.carjonline.org Abdominal Imaging / Imagerie abdominale A Review of Pneumatosis Intestinalis in the Setting of Systemic Cancer Treatments, Including Tyrosine Kinase Inhibitors Niranjan Vijayakanthan, MDa,*, Kavita Dhamanaskar, MD, FRCPCb,*, Lori Stewart, MD, FRCPCb, Jodie Connolly, RNc, Brian Leber, MD, FRCPCd,e, Irwin Walker, MD, FRCPCd,e, Michael Trus, MD, PhD, FRCPCd,f,y aThe Michael G. DeGroote School of Medicine, McMaster University, Hamilton, Ontario, Canada bDepartment of Radiology, Juravinski Hospital, Hamilton, Ontario, Canada cDepartment of Nursing, Hamilton General Hospital, Hamilton, Ontario, Canada dDivision of Hematology, Hamilton Health Sciences, Hamilton, Ontario, Canada eDepartment of Medicine, McMaster University, Hamilton, Ontario, Canada fDepartment of Pathology and Molecular Medicine, Hamilton Health Sciences, Hamilton, Ontario, Canada Abstract Purpose: Pneumatosis intestinalis is a radiologic diagnosis that manifests in a variety of clinical settings. We report 4 cases of pneumatosis intestinalis in patients undergoing cancer treatments that included cytotoxic agents and/or tyrosine kinase inhibitors. These reports aim to provide insight into the clinical interpretation and pathogenesis of pneumatosis intestinalis in the setting of cancer treatments and demonstrate a potential association with tyrosine kinase inhibitors. Methods: -

Abdominal Masses in Gynecology
11 Abdominal Masses in Gynecology Pubudu Pathiraja INTRODUCTION Some pelvic masses typically present only sono- graphically, for example: Abdominal or pelvic mass can present at any age of life. In a random sample of 335 asymptomatic • Endometrioma women aged 25–40 years, the point prevalence of • Hydro-/pyosalpinx an adnexal lesion on ultrasound examination was • Sero-/hematometra 1 7.8% (prevalence of ovarian cysts 6.6%) . Preva- If you diagnose a mass in a woman’s lower abdo- lence of pelvic masses in postmenopausal women men you must decide on: can vary widely from 2.5%2 to 10%3 in postmeno- • Which organ the tumor arises from (organ pausal women. specificity). Lower abdominal masses can be A woman presenting with an abdominal or divided according to their anatomical origin in pelvic mass might complain of various symptoms genital and extragenital masses (Table 2). but a significant majority will have no guiding/ • What kind of tumor you are dealing with ( entity, obvious symptoms at presentation although the i.e. malignant or benign). final diagnosis may be life-threatening. The abdo- men can be distended by either a solid or cystic Table 1 Common gynecological causes of free fluid in mass or by free fluid in the abdomen. To distin- the abdomen guish between masses and free fluid you must examine the patient and sometimes you will need Free See to perform an ultrasound as described in Chapter 1. fluid Cause Chapter: Blood Ectopic pregnancy 12 ABDOMINAL MASSES VERSUS A Pus Tubo-ovarian abscess/PID/peritonitis 5 DISTENDED ABDOMEN CAUSED BY FLUID Infected miscarriage 2, 13 Free fluid in the abdomen can either be ascites, Ascites Peritonitis, carcinomatosis (cancer) 26, blood, chylus or pus. -

Frank Rectal Bleeding: a Prospective Study of Causes in Patients Over the Age of 40
Postgraduate Medical Journal (1988) 64, 364-368 Postgrad Med J: first published as 10.1136/pgmj.64.751.364 on 1 May 1988. Downloaded from Frank rectal bleeding: a prospective study of causes in patients over the age of 40 P.S.Y. Cheung, S.K.C. Wong, J. Boey and C.K. Lai Department of Surgery, University of Hong Kong, Queen Mary Hospital, Hong Kong Summary: In order to determine the frequency of tumours in patients presenting with frank rectal bleeding, a prospective study was carried out in 337 patients over the age of 40 attending our Proctology Clinic. After making a clinical diagnosis, flexible sigmoidoscopy followed by barium enema was performed. Excluding 7 digitally palpable rectal cancers, there were 30 cancers (9.1%), 34 polyps (10.3%), 7 proctocolitis (2.1%) and 25 diverticula (7.6%) detected, giving a total diagnostic yield of 29.1%. A clinical diagnosis of bleeding sources other than haemorrhoids was made in 80% of the cancers. Flexible sigmoidoscopy detected 93.3% of the cancers and 88.2% of the polyps. Barium enema diagnosed the remaining cancers and polyps but there was a false-positive rate of 3.8%. From our results, we conclude that patients with frank rectal bleeding should be screened routinely for left-colon cancer irrespective of the clinical diagnosis. The flexible sigmoidoscope is a quick and useful tool. However, barium enema should be recommended to individuals with strong clinical suspicion of rectal bleeding other than haemorrhoids. Introduction copyright. Colorectal cancer is one of the commonest internal The purpose of this study was to evaluate malignancies in both sexes. -

Bowel Obstruction and Pelvic Mass
what-sobel_Layout 1 16/03/11 10:57 AM Page 686 Practice CMAJ What is your call? Bowel obstruction and pelvic mass Ally Murji MD, Mara L. Sobel MSc MD Competing interests: None 42-year-old woman presented to the Given the findings of bowel obstruction and a declared. emergency department with a four-day rectosigmoid mass in our patient, we suspected This article has been peer A history of severe constipation, bilious colorectal carcinoma. We proceeded with (c) a reviewed. vomiting and colicky abdominal pain. Her symp- colonoscopy, with the intent of obtaining a tissue Correspondence to: toms started with the onset of menses but dif- sample for diagnosis. Surprisingly, no mucosal Dr. Mara L. Sobel, fered from the crampy dysmenorrhea for which lesions were found. However, at the obstruction [email protected] she had taken over-the-counter analgesics for site, the mucosa was edematous with an a cute CMAJ 2011. DOI:10.1503 many years. She had no history of bloody or angulation of the sigmoid colon, compatible /cmaj.091851 black stool, or changes in her bowel function. with extra luminal compression. These findings, Apart from a 20 pack-year smoking history, her together with the previous imaging results, medical, surgical and family histories were non- pointed to external compression of the bowel contributory. from an adnexal mass or an intramural gastro - On examination, she was afebrile, and her intestinal lesion. As part of the recommended abdomen was soft but distended, with hyperac- workup for a pelvic or ovarian mass,1 we ordered tive, high-pitched bowel sounds.