21 CFR Ch. I (4–1–12 Edition) § 172.826
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

4.0 Product: Croustilis® Brand: SAF Pro® Date: 09/27/17
Document: 620-TDS-119 TECHNICAL DATA SHEET Revision: 4.0 Product: Croustilis® Brand: SAF Pro® Date: 09/27/17 Product Description As a dough conditioner, SAF Pro® Croustilis® reduces the formation of blisters and enables the successful production of bakery products. Regulatory Product is produced from materials that are wholesome and free from extraneous matter. Product is prepared following Good Manufacturing Practices, and complies with the Federal Food, Drug and Cosmetic Act of 1938, as amended and published in the CFR. Lesaffre Yeast Corporation is the US importer of record for SAF Pro Dough Conditioners manufactured by LIS- France, Cerences, France. LIS-France is included in Lesaffre Yeast Corporation’s Foreign Supplier Verification Program. Ingredient Statement Enriched Wheat Flour, Monoglycerides of Fatty Acids, Ascorbic Acid, Enzymes Manufacturing Process Blending, packaging Applications Baguettes, bagels, pizza crusts, mixes, frozen dough, par baked, retarded doughs, no-time doughs Usage Add directly to the flour at 0.3 – 0.5% of the total flour weight Item Numbers / Packaging Format / Grind / UPC Item Net Wt. Package Form UPC - Case Number 27201 10 kg. Poly-lined corrugated box Powder 0 17929 27201 2 Import / Export Tariff code 2106.90.99.98 Product Specifications Parameter Specification Test Method Ascorbic Acid 2.16-2.64% Internal Method Phc022 Salmonella Negative / 25 gm SMS (BIOMERIEUX), validated AFNOR # AES 10/04 - 05/04 7475 West Main St. Milwaukee, WI 53214 Document: 620-TDS-119 TECHNICAL DATA SHEET Revision: 4.0 Product: Croustilis® Brand: SAF Pro® Date: 09/27/17 Package / Pallet Dimensions Item Case Pallets L W H Cu. Tiers Units/ Cases/ L W H Cu. -

Copyright Statement This Copy of the Thesis Has Been Supplied on The
University of Plymouth PEARL https://pearl.plymouth.ac.uk 04 University of Plymouth Research Theses 01 Research Theses Main Collection 2017 Lactic acid bacteria as bio-preservatives in bakery Role of sourdough systems in the quality, safety and shelf life of bread Koy, Rebaz http://hdl.handle.net/10026.1/9828 University of Plymouth All content in PEARL is protected by copyright law. Author manuscripts are made available in accordance with publisher policies. Please cite only the published version using the details provided on the item record or document. In the absence of an open licence (e.g. Creative Commons), permissions for further reuse of content should be sought from the publisher or author. Copyright Statement This copy of the thesis has been supplied on the condition that anyone who consults it is understood to recognise that its copyright rests with its author and that no quotation from the thesis and no information derived from it may be published without the author’s prior consent. Lactic acid bacteria as bio-preservatives in bakery – Role of sourdough systems in the quality, safety and shelf life of bread by Rebaz Aswad Mirza Koy A thesis submitted to Plymouth University in partial fulfilment for the degree of DOCTOR OF PHILOSOPHY School of Biological and Marine Sciences Faculty of Science and Engineering August 2017 Lactic acid bacteria as bio-preservatives in bakery – Role of sourdough systems in the quality, safety and shelf life of bread Rebaz Aswad Mirza Koy Abstract Microbial contamination and survival during storage of bread are a cause of both health concerns and economic losses. -

Niaproof ® Calcium Stearoyl Lactylate
Technical Data Sheet Ref.: 2019_02v3 Niaproof ® Calcium Stearoyl Lactylate Niaproof® Calcium Stearoyl Lactylate is to exceed 0.5 parts for each 100 parts by offered as a white to cream colored powder weight of flour used. CSL is especially grade. preferred in lean hearth bread type formulations as a dough strengthener. PRODUCT PROPERTIES It is also used as a whipping agent in liquid and frozen egg white at a level not to Chemical Calcium Stearoyl-2- exceed 0.05 percent. In dried egg white at a name Lactylate level not to exceed 0.5 percent. In whipped Formula C48H86CaO12 vegetable oil topping at a level not to exceed 0.3 percent of the weight of the finished White to cream colored whipped vegetable oil topping. Product form powder with a slightly Niaproof CSL is used as a conditioning sweet (caramel) odor agent in dehydrated potatoes in an amount Molecular not to exceed 0.5 percent by weight thereof. 895.27 g/mol weight Legislation CAS No. 5793-94-2 Niaproof Calcium Stearoyl Lactylate EINECS No. 227-335-7 complies with the Food Chemical Codex (FCC). It is a non-toxic additive permitted for HS-code EU 2915.70 direct addition to food for human HS-code US 2915.70 consumption as listed in 21 CFR 172.844. Niaproof CSL is also an approved food Flash point 222 °C additive according to EU with E-number Solubility in Sparingly soluble E482. Water Please check local legislation for the exact dosage levels and allowed applications. Stability Applications Niaproof Calcium Stearoyl Lactylate is stable for 2 years from date of manufacture. -

Introduction to Baking and Pastries Chef Tammy Rink with William R
Introduction to Baking and Pastries Chef Tammy Rink With William R. Thibodeaux PH.D. ii | Introduction to Baking and Pastries Introduction to Baking and Pastries | iii Introduction to Baking and Pastries Chef Tammy Rink With William R. Thibodeaux PH.D. iv | Introduction to Baking and Pastries Introduction to Baking and Pastries | v Contents Preface: ix Introduction to Baking and Pastries Topic 1: Baking and Pastry Equipment Topic 2: Dry Ingredients 13 Topic 3: Quick Breads 23 Topic 4: Yeast Doughs 27 Topic 5: Pastry Doughs 33 Topic 6: Custards 37 Topic 7: Cake & Buttercreams 41 Topic 8: Pie Doughs & Ice Cream 49 Topic 9: Mousses, Bavarians and Soufflés 53 Topic 10: Cookies 56 Notes: 57 Glossary: 59 Appendix: 79 Kitchen Weights & Measures 81 Measurement and conversion charts 83 Cake Terms – Icing, decorating, accessories 85 Professional Associations 89 vi | Introduction to Baking and Pastries Introduction to Baking and Pastries | vii Limit of Liability/disclaimer of warranty and Safety: The user is expressly advised to consider and use all safety precautions described in this book or that might be indicated by undertaking the activities described in this book. Common sense must also be used to avoid all potential hazards and, in particular, to take relevant safety precautions concerning likely or known hazards involving food preparation, or in the use of the procedures described in this book. In addition, while many rules and safety precautions have been noted throughout the book, users should always have adult supervision and assistance when working in a kitchen or lab. Any use of or reliance upon this book is at the user's own risk. -

Two Effects of Surfactants in Bread
TWO EFFECTS OF SURFACTANTS IN SREAD I. AIR INCORPORATION IN BREAD DOUGH II. A MECHANISM FOR SHORTENING IMPROVEMENT OF LOAF VOLUME by RICHARD C. JUNGE B.S., Kansas State University, 1977 A MASTER'S THESIS submitted in partial fulfillment of the requirements for the degree MASTER OF SCIENCE Department of Grain Science and Industry KANSAS STATE UNIVERSITY Manhattan, Kansas 1980 Approved by (Major Professor) * Spec. Coll L-D \no ^ygS TABLE OF CONTENTS C 2. Page CHAPTER I. Incorporation of Air in Bread Dough 1 INTRODUCTION 2 LITERATURE REVIEW 3 Dough Density 3 Electron Microscopy of Dough A The Functions of Surfactants in Breadmaking 6 MATERIALS AND METHODS 7 Flour 7 Surfactants 7 Straight Dough Formula and Procedure 7 Dough Density Procedure 9 Scanning Electron Microscopy of Dough 9 RESULTS AND DISCUSSION 10 CONCLUSION 26 LITERATURE CITED 31 CHAPTER II. A Mechanism for Shortening Improvement of Loaf Volume 33 INTRODUCTION 34 LITERATURE REVIEW 35 Release of Carbon Dioxide from Dough During Bread Baking 35 The Effect of Temperature on Dough Properties 36 Starch Gelatinization During Baking 37 ii Page MATERIALS AND METHODS 39 Flour 39 Straight Dough Formula and Procedure 39 Carbon Dioxide Collection 39 Carbon Dioxide Quantification 40 Resistance Baking Oven 45 Photomicrographs 45 Pressure Measurements 45 Flour Defatting 45 RESULTS AND DISCUSSION 48 Oven Baking 48 Electric Resistance Oven 51 Effect of Shortening 51 Effect of Surfactants 57 Defatted Flours 66 Effect of Protein Quality 66 Measurement of Pressure in Dough 74 Effect of Oxidants 77 LITERATURE CITED 80 ACKNOWLEDGEMENTS 82 CHAPTER 1: Incorporation of Air in Bread Dough. -

(12) Patent Application Publication (10) Pub. No.: US 2004/0146601A1 Oszlanyi Et Al
US 2004O1466O1A1 (19) United States (12) Patent Application Publication (10) Pub. No.: US 2004/0146601A1 OSzlanyi et al. (43) Pub. Date: Jul. 29, 2004 (54) DOUGH CONDITIONER (52) U.S. Cl. ................................................................ 426/18 (76) Inventors: Antal G. Oszlanyi, Mooresville, NC (US); Azarel Nieves, Lafayette, LA (57) ABSTRACT (US) Correspondence Address: A method of improving the properties of dough and the PATTON BOGGS LLP quality of bread by adding to the flour a versatile dough SYSPARK DRIVE conditioner which includes an enzyme preparation consist MCLEAN, VA 22102 (US) ing of an amylase, hemicellulase, and a lipase, oxidizing agent containing ascorbic acid and azodicarbonamide, and a (21) Appl. No.: 10/352,096 Sulfhydryl reducing agent. The enzyme preparation has the advantageous effect of lowering the percent by weight of (22) Filed: Jan. 28, 2003 additives relative to the flour, while improving the ability to Publication Classification process the dough and the properties of the final baking product. The invention is effective in a variety of baking (51) Int. Cl. .................................................... A23L 1/10 methods and all types of yeast leavened products. US 2004/0146601 A1 Jul. 29, 2004 DOUGH CONDITIONER dough of Similar or Superior quality. The conditioner is remarkably versatile and capable of performing in a variety BACKGROUND OF THE INVENTION of baking methods without a noticeable difference in crumb Structure or loaf Volume. 0001) 1. Field of the Invention 0007 Accordingly, it is an object of the present invention 0002 The present invention relates to a dough condi to provide an effective replacement for potassium bromate. tioner and to a method of using the conditioner to improve bread quality. -

21 CFR Ch. I (4–1–16 Edition)
§ 137.200 21 CFR Ch. I (4–1–16 Edition) codeloflfederallregulations/ ‘‘Fungal alpha-amylase,’’ ‘‘Fungal a- ibrllocations.html. amylase’’, ‘‘Enzyme’’, or ‘‘Enzyme [42 FR 14402, Mar. 15, 1977, as amended at 47 added for improved baking’’. When any FR 11827, Mar. 19, 1982; 49 FR 10097, Mar. 19, optional bleaching ingredient is used, 1984; 54 FR 24894, June 12, 1989] the label shall bear the word ‘‘Bleached’’. Wherever the name of the § 137.200 Whole wheat flour. food appears on the label so conspicu- (a) Whole wheat flour, graham flour, ously as to be easily seen under cus- entire wheat flour is the food prepared tomary conditions of purchase, the by so grinding cleaned wheat, other word ‘‘Bleached’’ shall immediately than durum wheat and red durum and conspicuously precede or follow wheat, that when tested by the method such name, without intervening writ- prescribed in paragraph (c)(2) of this ten, printed, or graphic matter; except section, not less than 90 percent passes that where such name is a part of a through a 2.36 mm (No. 8) sieve and not trademark or brand, other written, less than 50 percent passes through a printed or graphic matter, which is 850 μm (No. 20) sieve. The proportions also a part of such trademark or brand, of the natural constituents of such may so intervene if the word wheat, other than moisture, remain ‘‘Bleached’’ is in such juxtaposition unaltered. To compensate for any nat- with such trademark or brand as to be ural deficiency of enzymes, malted conspicuously related to such name. -
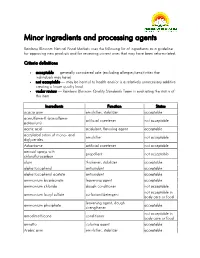
Minor Ingredients and Processing Agents
Minor ingredients and processing agents Rainbow Blossom Natural Food Markets uses the following list of ingredients as a guideline for approving new products and for reviewing current ones that may have been reformulated. Criteria definitions acceptable — generally considered safe (excluding allergies/sensitivities that individuals may have) not acceptable — may be harmul to health and/or is a relatively unnecessary additive creating a lower quality food under review — Rainbow Blossom Quality Standards Team is evaluating the status of this item Ingredients Function Status acacia gum emulsifier, stabilizer acceptable acesulfame-K (acesulfame- artificial sweetener not acceptable potassium) acetic acid acidulant, flavoring agent acceptable acetylated esters of mono- and emulsifier not acceptable diglycerides Advantame artificial sweetener not acceptable aerosol sprays with propellant not acceptable chloroflurocarbon algin thickener, stabilizer acceptable alpha tocopherol antioxidant acceptable alpha tocopherol acetate antioxidant acceptable ammonium bicarbonate leavening agent acceptable ammonium chloride dough conditioner not acceptable not acceptable in ammonium lauryl sulfate surfactant/detergent body care or food leavening agent, dough ammonium phosphate acceptable strengthener not acceptable in amodimethicone conditioner body care or food annatto coloring agent acceptable Arabic gum emulsifier, stabilizer acceptable Ingredients Function Status artificial colors (red #3, red # 40, yellow #5, yellow #6, blue #1, blue coloring agent -

Pgdbst – 05: Bread Industry and Processes
POST GRADUATE DIPLOMA IN BAKERY SCIENCE AND TECHNOLOGY PGDBST – 05 BREAD INDUSTRY AND PROCESSES DIRECTORATE OF DISTANCE EDUCATION GURU JAMBHESHWAR UNIVERSITY OF SCIENCE AND TECHNOLOGY HISAR – 125 001 2 PGDBST- 05 B.S.Khatkar UNIT 1: BREAD MAKING PROCESS STRUCTURE 1.0 OBJECTIVES 1.1 STATUS OF BAKING INDUSTRY 1.2 BREAD FORMULATION 1.3 BREAD MAKING PROCEDURE 1.4 FUNCTIONS OF MIXING 1.5 TYPES OF MIXERS 1.6 FUNCTIONS OF MOULDING AND DIVIDING 1.7 FUNCTIONS OF PROVING 1.8 CHANGES DURING MIXING, FERMENTATION AND BAKING 1.9 SUMMARY 1.10 KEY WORDS 1.11 SELF ASSESSMENT QUESTIONS 1.12 SUGGESTED READINGS 3 1.0 OBJECTIVES Thorough study of this unit will enable the reader to understand: • Status of baking industry • Bread making procedure • Types of mixers • Functions of mixing, moulding, dividing and proving • Changes during mixing, fermentation and baking 1.1 STATUS OF BAKING INDUSTRY India is the 2nd largest wheat producing country in the world next only to China. The present production of wheat in India is about 72 million tonnes indicating 6-fold increase in the three decade due to onset of green revolution. The five major wheat producing states in India are U.P., Punjab, Haryana, Bihar and Himachal Pradesh. Unlike in other economically developed nations, bulk of the wheat produced in our country is processed into whole wheat flour for use in various traditional products. About 10 per cent of the total wheat produced is processed into different products like maida, suji, atta, etc. in roller flour mill, which forms the main raw material for bakery and pasta industry. -
![United States Patent [19] [11] Patent Number: 4,643,900](https://docslib.b-cdn.net/cover/0885/united-states-patent-19-11-patent-number-4-643-900-2010885.webp)
United States Patent [19] [11] Patent Number: 4,643,900
United States Patent [19] [11] Patent Number: 4,643,900 Porter [45] Date of Patent: Feb. 17 3 1987 [54] METHOD FOR MAKING BAKERY PRODUCTS OTHER PUBLICATIONS _ , Furia et a1, Fenaroli’s Handbook of Flavor Ingredients, [75] Invenm“ R” W- PM“, 131m“, Cahf- vol. I, 2nd ed., 1975, CRC Press: Cleveland, pp. [73] Assignee: Basic American Foods, San 196_l_97' _ _ _ Francisco’ Calif' Pruthl Spices and Condiments, 1980, Academic Press: New York, pp. 200-201. [21] Appl' No': “L707 Primary Examiner-Joseph Golian [22] Filed: Oct. 17, 1984 Attorney, Agent, or Firm-Sandler & Greenblum [51] Int. Cl.‘‘ ............................................. .. A21D 2/36 [57] ABSTRACT [52] US. Cl. .................................... .. 426/21; 426/658; A method for producing bakery products is provided _ 426/549; 426/19 wherein a dough conditioner composition of allium [58] Field of Search ................. .. 426/549, 653, 19, 21, material is incorporated into dough formulations‘ in_ 426/62 eluding flour, water and leavening agents. The allium [56] References Cited material is used in effective amounts to function in re ducing the mix-time required to develop a dough, in U-s- PATENT DOCUMENTS creasing the absorption of water by the flour during 2,883,285 4/ 1959 Lubig . dough development, and increasing the extensibility of 3,053,666 9/1962 Henika et al. the dough. The preferred allium material used in the 3,537,863 11/1970 Sinnott . preparation of the dough improving composition is 3,556,805 1/1971 Conn et al. garlic, more preferably in a dehydrated form and in 3,594,131 7/ 1971 Alp!" - combination with an inert organic material. -

2018 Food Ingredient List ***(Packaged Food Will Show Ingredient List on Package)
2018 FOOD INGREDIENT LIST ***(PACKAGED FOOD WILL SHOW INGREDIENT LIST ON PACKAGE) Alder Smoked Salmon Chowder (served for lunch) Water, potatoes, chowder mix, smoked salmon, canola oil, flour modified corn starch, pesto sauce, alder seasoning, salt dehydrated onions, lemon juice, dehydrated red bell peppers, dehydrated celery, parmesan cheese base, romano cheese base, granulated garlic, chowder spice, black pepper. CONTAINS FISH, WHEAT, GLUTEN, MILK, SOY, COCONUT, TREE NUT Vegetable Chili (served for lunch) Gluten Free Water, diced tomatoes in juice (tomatoes, tomato juice, citric acid, calcium chloride), pinto beans, onions, light red kidney beans, vegetable soup base (carrot, celery, onion, tomato paste, corn oil, yeast extract, potato flour, salt, onion powder, garlic powder, flavoring), contains less than 2% of the following: tomato paste (tomatoes, citric aced), red bell peppers, carrots, green bell peppers, chopped garlic (garlic, water), salt, modified corn starch, spices, chili pepper, cilantro, pepper sauce (vinegar, red pepper, salt). ALLERGENS: NONE Julienne Vegetable Blend Salad (served for lunch) Gluten Free Broccoli, cauliflower, carrots, beets, brussels sprouts, kale, radicchio. Welch’s Mixed Fruit Snack (served for lunch) Gluten Free Fruit puree (grape, peach, orange, strawberry and raspberry), water, corn syrup, sugar, inulin (from chicory root), modified corn starch, gelatin, concord grape juice from concentrate, citric acid, lactic acid, ascorbic acid (vitamin C), natural and artificial flavors, alpha tocopherol acetate (vitamin E) vitamin A Palmitate, sodium citrate, coconut oil, carnauba wax, annatto (color), turmeric (color), Red 40, Blue 1. Multigrain Bread Roll (served for lunch) Water, whole wheat flour, enriched flour (wheat flour, malted barley flour, niacin, ferrous sulfate, thiamin mononitrate, riboflavin, folic acid), multigrain blend (rye flour, rye flakes, steel cut oats, millet, flaxseeds, cracked wheat, wheat gluten, sunflower seeds, sesame seeds, rye meal, barley flakes). -

The Effects of Additives on Dough Rheological
THE EFFECTS OF ADDITIVES ON DOUGH RHEOLOGICAL PROPERTIES OF PRE-PROOFED FROZEN DOUGH AND BAKING QUALITY OF BREAD STICKS By JUNTANEE URIYAPONGSON Bachelor of Science Kasetsart University Bangkok, Thailand 1983 Master of Science North Dakota State University Fargo, North Dakota 1993 Submitted to the Faculty of the Graduate College of the Oklahoma State University in partial fulfillment of the requirements for the Degree of DOCTOR OF PHILOSOPHY May,2002 THE EFFECTS OF ADDITIVES ON DOUGH RHEOLOGICAL PROPERTIES OF PRE-PROOFED FROZEN DOUGH AND BAKING QUALITY OF BREAD STICKS Thesis Approved: Thesis Adviser ~l~• ii ACKNOWLEDGMENTS I would like to express my sincere appreciation to my advisor, Dr. Patricia Rayas for her support, excellent supervision, guidance, and wonderful friendship. I am also thankful to my committee members, Dr. Stanley E. Gilliland, Dr. Gerald H. Brusewitz, Dr. William McGlynn and former committee member Dr. Lowell Satterlee, who retired before my thesis completed, for their support and suggestions on my research work, and to Dr. Carla L. Goad for her assistance and suggestion in statistical analysis ofmy data. I am also thankful to David Moe, pilot plant manager for his assistance and suggestion for the trouble shooting of equipment and instrument problems, to Phoebe Doss for SEM assistance, to Frank McKee for his support and giving me food industry experience, to Dr. Inteaz Alli for his support and giving me experience in Quality Assurance in food industries, his caress, and been very good friend, to Dr. Mahendra Kavdia for technical assistance in computer work and my friends for their good companies and different life during my study.