NIH Multi-IC (NIAID/NCI/NIGMS/NIBIB) Small Business Workshop 2016 September 7 & 8, 2016 Location: University of Texas San Antonio/University of Texas Health Science Center at San Antonio
Wednesday, September 7, 2016
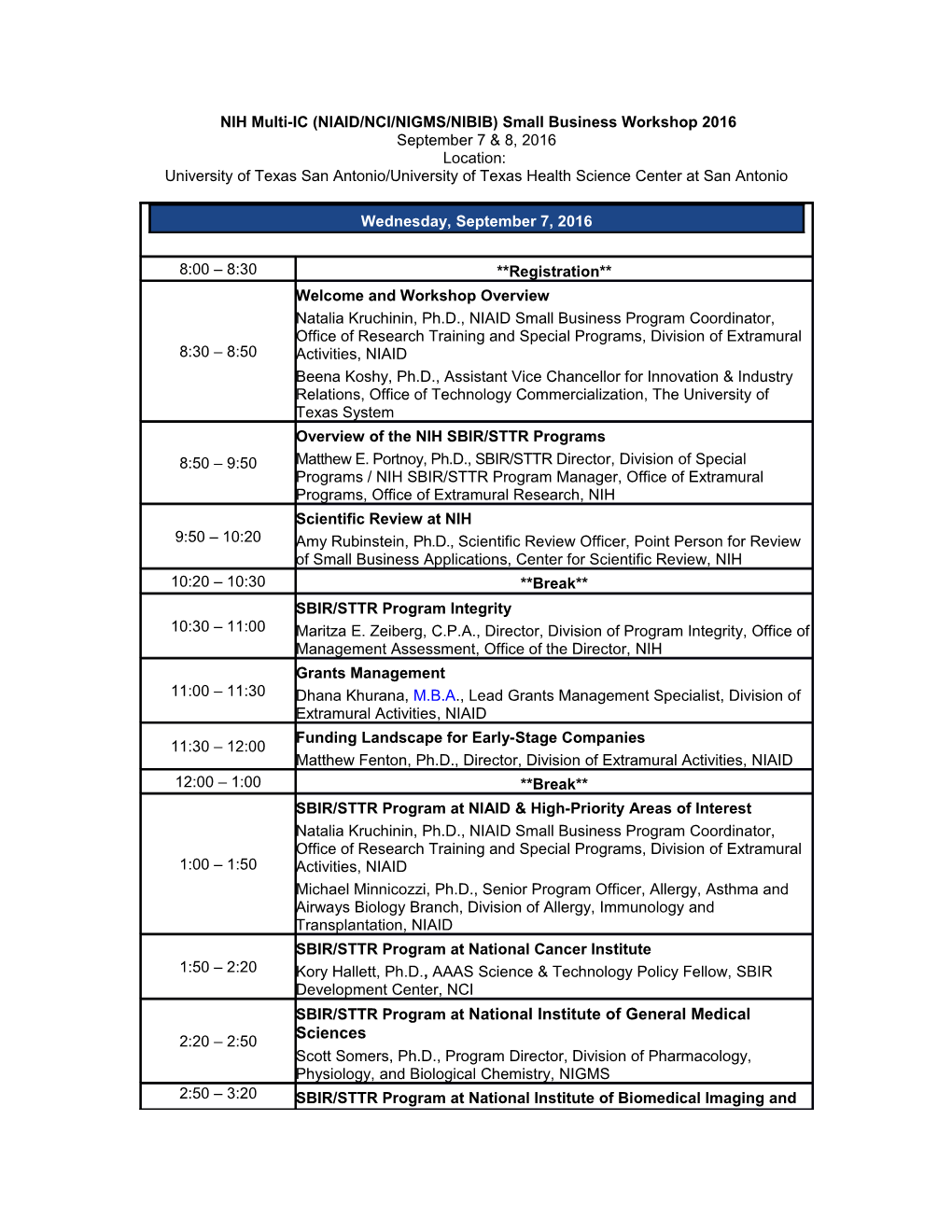
8:00 – 8:30 **Registration** Welcome and Workshop Overview Natalia Kruchinin, Ph.D., NIAID Small Business Program Coordinator, Office of Research Training and Special Programs, Division of Extramural 8:30 – 8:50 Activities, NIAID Beena Koshy, Ph.D., Assistant Vice Chancellor for Innovation & Industry Relations, Office of Technology Commercialization, The University of Texas System Overview of the NIH SBIR/STTR Programs 8:50 – 9:50 Matthew E. Portnoy, Ph.D., SBIR/STTR Director, Division of Special Programs / NIH SBIR/STTR Program Manager, Office of Extramural Programs, Office of Extramural Research, NIH Scientific Review at NIH 9:50 – 10:20 Amy Rubinstein, Ph.D., Scientific Review Officer, Point Person for Review of Small Business Applications, Center for Scientific Review, NIH 10:20 – 10:30 **Break** SBIR/STTR Program Integrity 10:30 – 11:00 Maritza E. Zeiberg, C.P.A., Director, Division of Program Integrity, Office of Management Assessment, Office of the Director, NIH Grants Management 11:00 – 11:30 Dhana Khurana, M.B.A., Lead Grants Management Specialist, Division of Extramural Activities, NIAID Funding Landscape for Early-Stage Companies 11:30 – 12:00 Matthew Fenton, Ph.D., Director, Division of Extramural Activities, NIAID 12:00 – 1:00 **Break** SBIR/STTR Program at NIAID & High-Priority Areas of Interest Natalia Kruchinin, Ph.D., NIAID Small Business Program Coordinator, Office of Research Training and Special Programs, Division of Extramural 1:00 – 1:50 Activities, NIAID Michael Minnicozzi, Ph.D., Senior Program Officer, Allergy, Asthma and Airways Biology Branch, Division of Allergy, Immunology and Transplantation, NIAID SBIR/STTR Program at National Cancer Institute 1:50 – 2:20 Kory Hallett, Ph.D., AAAS Science & Technology Policy Fellow, SBIR Development Center, NCI SBIR/STTR Program at National Institute of General Medical Sciences 2:20 – 2:50 Scott Somers, Ph.D., Program Director, Division of Pharmacology, Physiology, and Biological Chemistry, NIGMS 2:50 – 3:20 SBIR/STTR Program at National Institute of Biomedical Imaging and Bioengineering (NIBIB)- Small Business Support for Medical Device Development Mr. Todd Merchak, Biomedical Engineer, NIBIB 3:20 – 3:30 **Break** The March Toward a Licensed PfSPZ Malaria Vaccine: A Journey Made Possible by SBIRs 3:30-4:00 Stephen L. Hoffman, M.D., D.T.M.H., D.Sc. (hon), FASTMH, CAPT, MC, USN (RET), Chief Executive and Scientific Officer, Sanaria, Inc. Writing a Competitive SBIR/STTR Phase I Application 4:00 – 4:30 Lisa M. Kurek, M.S., Managing Partner, BBC Entrepreneurial Training & Consulting, LLC
One-On-One Meetings with Government Staff 4:30 – 5:30 NIAID, NIGMS, NCI, NIBIB representatives Thursday, September 8, 2016
8:30 – 9:00 **Registration** The Path from Discovery to Market 9:00 – 9:50 Andrey J. Zarur, Ph.D., Managing General Partner, Kodiak Venture Partners SBIR-funded Product: Development, Validation, Launch 9:50 – 10:40 David Beylin, M.S., M.B.A., CEO, BLN Scientific, LLC How to Pitch Your Business to Investors? 10:40 – 11:30 Liddy Karter, M.B.A., Managing Director, Enhanced Capital Partners 11:30 – 11:40 **Break** Panel: Diversifying NIH’s SBIR/STTR Program Moderator: Paula Strickland, Ph.D., M.P.H., Director, Office of Research Training and Special Programs, DEA, NIAID Panel members: 11:40-12:40 Matthew E. Portnoy, Ph.D., NIH SBIR/STTR Program Coordinator David Beylin, M.S., M.B.A., CEO, BLN Scientific, LLC Diane Adger-Johnson, Health Science Program Officer, ORTSP, DEA, NIAID Kory Hallett, Ph.D., AAAS Science & Technology Policy Fellow, SBIR Development Center, NCI 12:40-1:40 **Break** Writing a Competitive SBIR/STTR Phase II Application 1:40 – 2:30 Lisa M. Kurek, M.S., Managing Partner, BBC Entrepreneurial Training & Consulting, LLC
Lessons Learned – Invest Your Time Wisely - by Yaso Biotechnology, 2:30-3:00 Inc. Mary F. Weitzel, M.B.A., CEO and President, Yaso Biotechnology, Inc. 3:00-3:20 Connecting the SBIR/STTR and I-Corps programs to achieve commercialization success across the University of Texas System Carlos A. Kemeny, Ph.D., M.B.A. Assistant Director, Office of Technology Commercialization, The University of Texas System TX Success story 3:20-3:50 Sarah Mayes, Ph.D., Director of R&D, Alafair Biosciences, Inc. Wrap-Up and Closing Remarks Natalia Kruchinin, Ph.D., NIAID Small Business Program Coordinator, Office of Research Training and Special Programs, Division of Extramural 3:50-4:00 Activities, NIAID Beena Koshy, Ph.D., Assistant Vice Chancellor for Innovation & Industry Relations, Office of Technology Commercialization, The University of Texas System