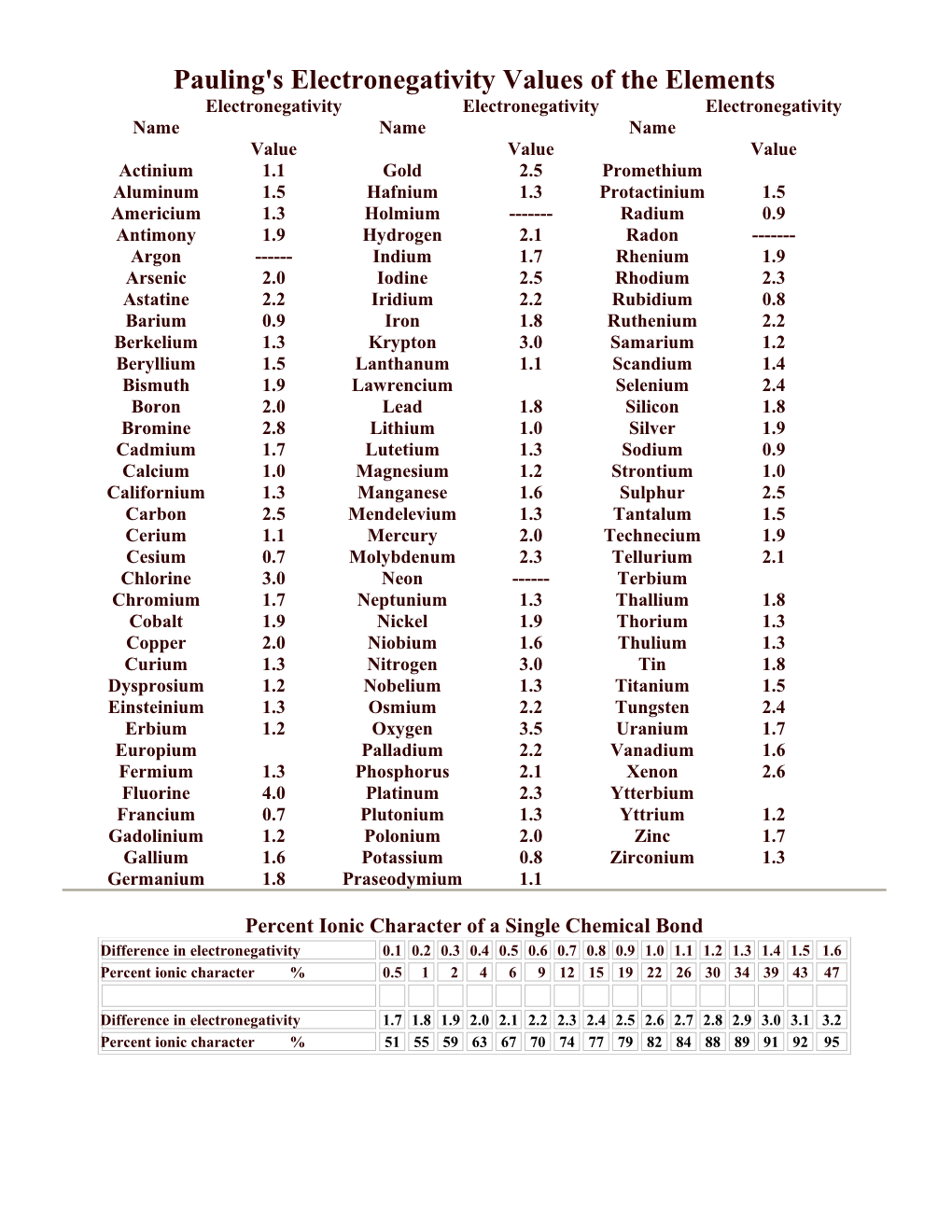
Pauling's Electronegativity Values of the Elements Electronegativity Electronegativity Electronegativity Name Name Name Value Value Value Actinium 1.1 Gold 2.5 Promethium Aluminum 1.5 Hafnium 1.3 Protactinium 1.5 Americium 1.3 Holmium ------Radium 0.9 Antimony 1.9 Hydrogen 2.1 Radon ------Argon ------Indium 1.7 Rhenium 1.9 Arsenic 2.0 Iodine 2.5 Rhodium 2.3 Astatine 2.2 Iridium 2.2 Rubidium 0.8 Barium 0.9 Iron 1.8 Ruthenium 2.2 Berkelium 1.3 Krypton 3.0 Samarium 1.2 Beryllium 1.5 Lanthanum 1.1 Scandium 1.4 Bismuth 1.9 Lawrencium Selenium 2.4 Boron 2.0 Lead 1.8 Silicon 1.8 Bromine 2.8 Lithium 1.0 Silver 1.9 Cadmium 1.7 Lutetium 1.3 Sodium 0.9 Calcium 1.0 Magnesium 1.2 Strontium 1.0 Californium 1.3 Manganese 1.6 Sulphur 2.5 Carbon 2.5 Mendelevium 1.3 Tantalum 1.5 Cerium 1.1 Mercury 2.0 Technecium 1.9 Cesium 0.7 Molybdenum 2.3 Tellurium 2.1 Chlorine 3.0 Neon ------Terbium Chromium 1.7 Neptunium 1.3 Thallium 1.8 Cobalt 1.9 Nickel 1.9 Thorium 1.3 Copper 2.0 Niobium 1.6 Thulium 1.3 Curium 1.3 Nitrogen 3.0 Tin 1.8 Dysprosium 1.2 Nobelium 1.3 Titanium 1.5 Einsteinium 1.3 Osmium 2.2 Tungsten 2.4 Erbium 1.2 Oxygen 3.5 Uranium 1.7 Europium Palladium 2.2 Vanadium 1.6 Fermium 1.3 Phosphorus 2.1 Xenon 2.6 Fluorine 4.0 Platinum 2.3 Ytterbium Francium 0.7 Plutonium 1.3 Yttrium 1.2 Gadolinium 1.2 Polonium 2.0 Zinc 1.7 Gallium 1.6 Potassium 0.8 Zirconium 1.3 Germanium 1.8 Praseodymium 1.1
Percent Ionic Character of a Single Chemical Bond Difference in electronegativity 0.1 0.2 0.3 0.4 0.5 0.6 0.7 0.8 0.9 1.0 1.1 1.2 1.3 1.4 1.5 1.6 Percent ionic character % 0.5 1 2 4 6 9 12 15 19 22 26 30 34 39 43 47
Difference in electronegativity 1.7 1.8 1.9 2.0 2.1 2.2 2.3 2.4 2.5 2.6 2.7 2.8 2.9 3.0 3.1 3.2 Percent ionic character % 51 55 59 63 67 70 74 77 79 82 84 88 89 91 92 95