Chapter 7 Worksheet (Practice)
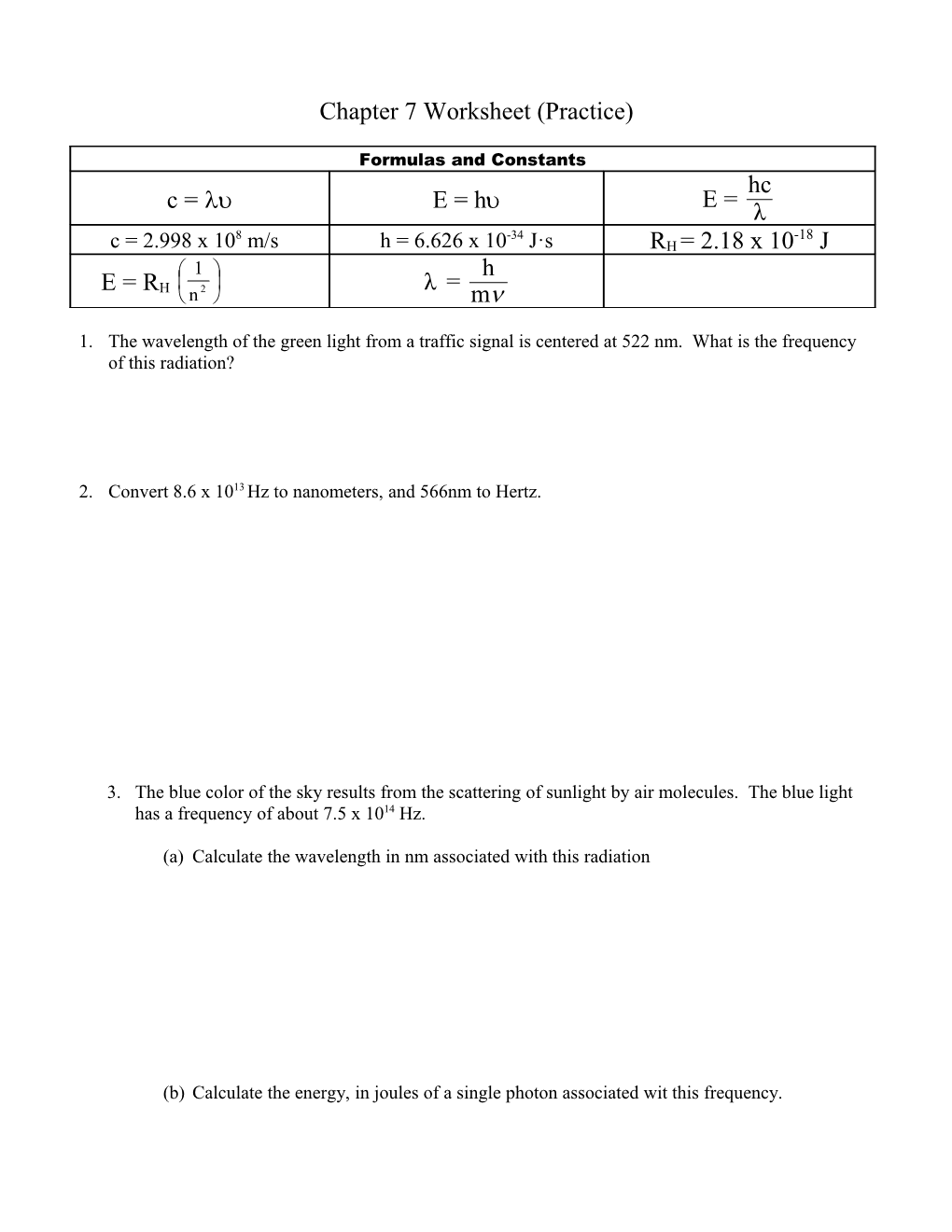
Formulas and Constants hc E = c = E = h λ 8 -34 -18 c = 2.998 x 10 m/s h = 6.626 x 10 J·s RH = 2.18 x 10 J 1 h E = R 2 λ = H n m
1. The wavelength of the green light from a traffic signal is centered at 522 nm. What is the frequency of this radiation?
2. Convert 8.6 x 1013 Hz to nanometers, and 566nm to Hertz.
3. The blue color of the sky results from the scattering of sunlight by air molecules. The blue light has a frequency of about 7.5 x 1014 Hz.
(a) Calculate the wavelength in nm associated with this radiation
(b) Calculate the energy, in joules of a single photon associated wit this frequency. 4. What is the de Broglie wavelength of a 12.4-g hummingbird flying at 1.20 x 102 mph?
5. Calculate the wavelength (in nanometers) of a photon emitted by a hydrogen atom when its electron drops from the n = 5 to the n = 3 state.
6. Indicate which of the following sets of quantum numbers are incorrect, and why.
(a) (1, 0, 1/2, -1/2)
(b) (3, 0, 0, +1/2)
(c) (3, 2, 1, 1)
7. Write the electron configurations for the atom and the ion it forms.
(a) O
(b) K
(c) Fe
(d) Cl
(e) N