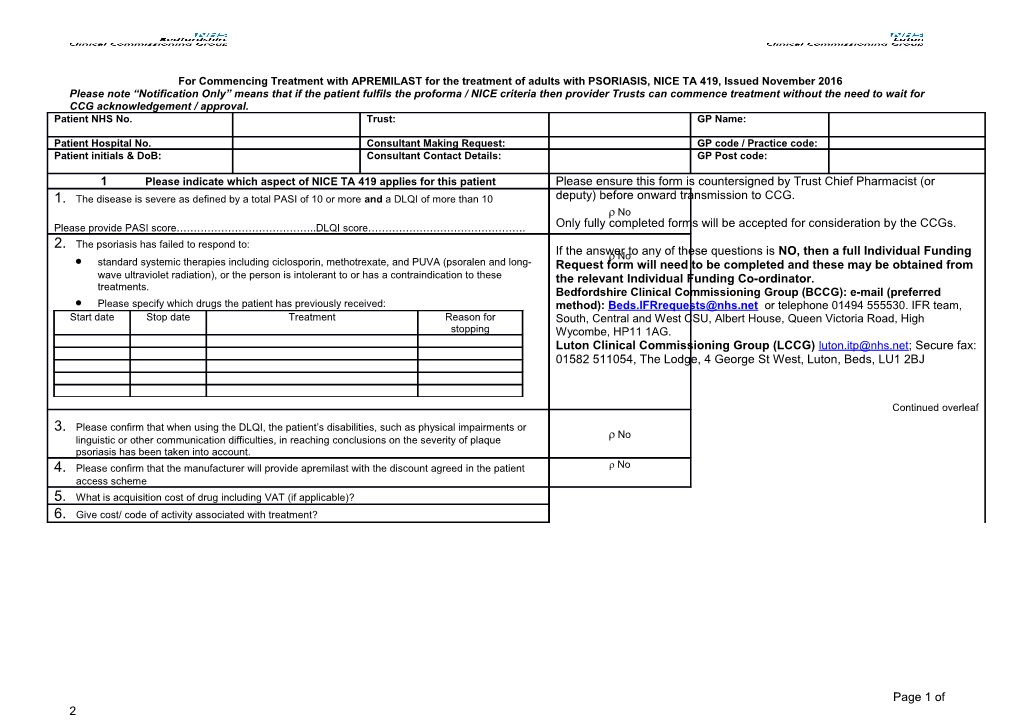
For Commencing Treatment with APREMILAST for the treatment of adults with PSORIASIS, NICE TA 419, Issued November 2016 Please note “Notification Only” means that if the patient fulfils the proforma / NICE criteria then provider Trusts can commence treatment without the need to wait for CCG acknowledgement / approval. Patient NHS No. Trust: GP Name:
Patient Hospital No. Consultant Making Request: GP code / Practice code: Patient initials & DoB: Consultant Contact Details: GP Post code:
1 Please indicate which aspect of NICE TA 419 applies for this patient Please ensure this form is countersigned by Trust Chief Pharmacist (or 1. The disease is severe as defined by a total PASI of 10 or more and a DLQI of more than 10 deputy) before onward transmission to CCG. No Please provide PASI score…………………………………..DLQI score………………………………………. Only fully completed forms will be accepted for consideration by the CCGs. 2. The psoriasis has failed to respond to: If the answer No to any of these questions is NO, then a full Individual Funding standard systemic therapies including ciclosporin, methotrexate, and PUVA (psoralen and long- Request form will need to be completed and these may be obtained from wave ultraviolet radiation), or the person is intolerant to or has a contraindication to these the relevant Individual Funding Co-ordinator. treatments. Bedfordshire Clinical Commissioning Group (BCCG): e-mail (preferred Please specify which drugs the patient has previously received: method): [email protected] or telephone 01494 555530. IFR team, Start date Stop date Treatment Reason for South, Central and West CSU, Albert House, Queen Victoria Road, High stopping Wycombe, HP11 1AG. Luton Clinical Commissioning Group (LCCG) [email protected]; Secure fax: 01582 511054, The Lodge, 4 George St West, Luton, Beds, LU1 2BJ
Continued overleaf 3. Please confirm that when using the DLQI, the patient’s disabilities, such as physical impairments or No linguistic or other communication difficulties, in reaching conclusions on the severity of plaque psoriasis has been taken into account. 4. Please confirm that the manufacturer will provide apremilast with the discount agreed in the patient No access scheme 5. What is acquisition cost of drug including VAT (if applicable)? 6. Give cost/ code of activity associated with treatment?
Page 1 of 2 7. For continuation therapy notification: Treatment should only be continued if the patient has responded to 16 weeks treatment, i.e. A 75% reduction in the PASI score from when treatment started (PASI 75) or a 50% reduction in the PASI score (PASI 50) and a 5-point reduction in the DLQI from when treatment started. Please choose one these options. The patient has shown an adequate response either: A 75% reduction in the PASI score from when treatment started (PASI 75) or a 50% reduction in the PASI score (PASI 50) and a five-point reduction in the DLQI from when treatment started. Please give scores to corroborate option selected: Pre-treatment PASI score: Pre-treatment DLQI score: Post-treatment PASI score: Post-treatment DLQI score: PASI score % reduction: DLQI score reduction:
Please confirm patient has not suffered severe drug related toxicity.
3 Trust contact e-mail in case of CCG query: 4 5 I confirm that the patient (or in the case of a minor or vulnerable adult where 6 Yes No the parent/guardian or legal carer) has given consent for the patient identifiable data on this form to be shared with the CCG Medicines Management / Optimisation or Contracts Team. This data may then be used 1. In the interests of the care of the patient 2. For clinical audit purposes 3. To validate against subsequent invoices. 7 Consultant Signature (electronic signature acceptable) 8 Trust Chief Pharmacist (or deputy) signature (electronic signature acceptable)
9 Date 10 Date
11 12 FOR CCG USE ONLY CCG notified of first 16 weeks treatment? Yes No CCG notified of treatment continuation after 16 Yes No weeks?
NICE Guidance TA419. Apremilast for treating moderate to severe plaque psoriasis, issued November 2016. 1.1 Apremilast is recommended as an option for treating chronic plaque psoriasis in adults whose disease has not responded to other systemic therapies, including ciclosporin, methotrexate and PUVA (psoralen and ultraviolet-A light), or when these treatments are contraindicated or not tolerated, only if: the disease is severe, as defined by a total Psoriasis Area Severity Index (PASI) of 10 or more and a Dermatology Life Quality Index (DLQI) of more than 10 treatment is stopped if the psoriasis has not responded adequately at 16 weeks; an adequate response is defined as: o a 75% reduction in the PASI score (PASI 75) from when treatment started or o a 50% reduction in the PASI score (PASI 50) and a 5-point reduction in DLQI from start of treatment
LCCG: This form should be returned to [email protected]; Secure fax: 01582 511054, The Lodge, 4 George St West, Luton, LU1 2BJ. BCCG: This form should be returned to: [email protected] (e-mail preferred method) or IFR team, South, Central and West CSU, Albert House, Queen Victoria Road, High Wycombe, HP11 1AG. D:\Docs\2018-04- 02\06a6a51494489ec964c87b7bf2d41b28.docx 2 of 3 the company provides apremilast with the discount agreed in the patient access scheme.
1.2 When using the DLQI, healthcare professionals should take into account any physical, sensory or learning disabilities, or communication difficulties, that could affect the responses to the DLQI and make any adjustments they consider appropriate.
LCCG: This form should be returned to [email protected]; Secure fax: 01582 511054, The Lodge, 4 George St West, Luton, LU1 2BJ. BCCG: This form should be returned to: [email protected] (e-mail preferred method) or IFR team, South, Central and West CSU, Albert House, Queen Victoria Road, High Wycombe, HP11 1AG. D:\Docs\2018-04- 02\06a6a51494489ec964c87b7bf2d41b28.docx 3 of 3