Homework No 1 Organic Chemistry A Date: 29/09/2015 Dr. Ramy Y. Morjan Student Name: I.D.
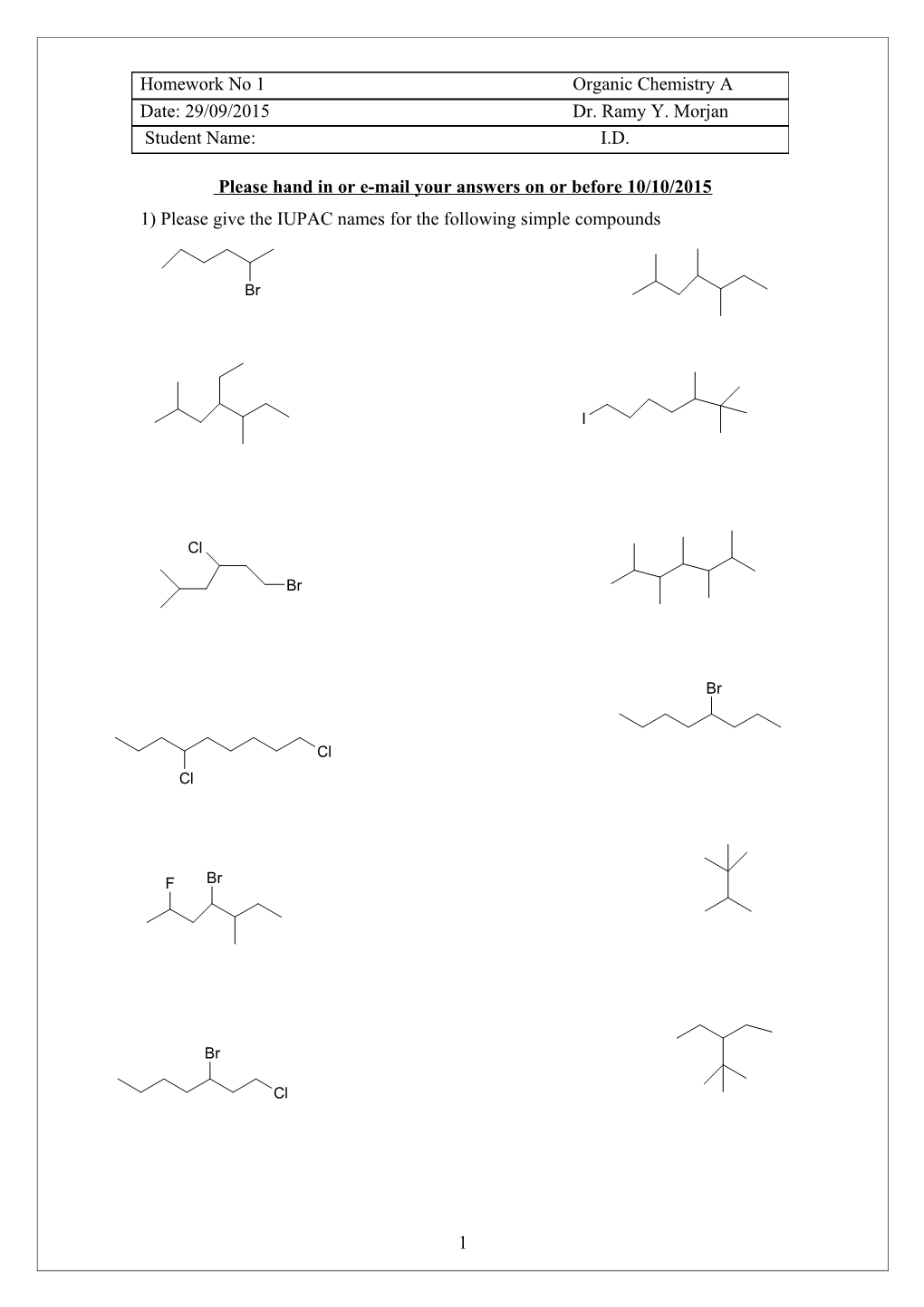
Please hand in or e-mail your answers on or before 10/10/2015 1) Please give the IUPAC names for the following simple compounds
Br
I
Cl
Br
Br
Cl Cl
F Br
Br
Cl
1 2) Please draw the chemical structure for the following compounds
a) 1,6-dichlorononane
b) 1-bromo-3-chloro-5-methylhexane
c) 1-iodo-5,6,6-trimethylheptane
d) 4-ethyl-2,5-dimethylheptane
e) 2,3,4,5,6-pentamethylheptane
f) 4-bromo-2-fluoro-5-methylheptane
g) 2,2,3-trimethylbutane
h) 3,5-diflorocyclohexane
i) 3-Bromocyclopentane
2 3) Compounds that have the same molecular formula but different chemical structures are called isomers. On the light of your understanding draw all the possible isomeric structures of C4H10. Do you think the isomers will have the same chemical and physical properties or no? Explain your answers!
4) What is the shape of s, p, sp orbitals (draw them) and what is the type of bonds that formed as a result of overlapping between s&s, s&p, s&sp3, p&p, sp3&sp3 (please show the all possible overlapping)
3 5) The reaction of propane with Cl2 in the present of sun light has produced two
isomeric products x, and y in different percentage yields.
I. Write the chemical structure for the two products and indicates which of them will be formed in a higher percentage than the other and explain why?
II. In light of your understanding to hybridization and orbitals shape and overlapping; draw a sketch for any of the two products to show each type of hybridization on the C atoms and how the δ bond is formed in terms of orbitals overlapping.
III. If the propane was reacted with Br2 in the present of light; how many product do you expect to have? If more than one product will be formed what is the percentage yield do you expect for each one and why?
4 6) Please underline the correct answer for the following questions
1. Arrange the following types of intermolecular forces in order of increasing strength. • Ion-Ion • Dipole-Dipole • Hydrogen Bonding • London Dispersion
2. Arrange the following compounds in the order you would think that their boiling points would be. (lowest first) Explain your order. • C10H22 • C2H6 • C8H18 • C4H10
3. Arrange the following compounds in the order that you think their boiling points would be. (lowest first)
• C2H5OH
• C3H8 • CH3Cl • H2O
3. Arrange the following compounds in the order that you think their solubility in water would be. (lowest first)
• C2H5OH
• C3H8 • CH3CH2CH2OH • C5H12
4. Arrange the following compounds in the order that you think their boiling points would be. (lowest first)
• Pentane • Isopentane • Butane • Isobutane
5 7) Write all the possible mono-halogenation products for the following reactions
Light
Br2
Light
Cl2
Light
Cl2
Light
Br2
8) Write all the possible dihalogenation products for the following reactions
Light
Br2
Light
Cl2
Light
Cl2
6 9) Which of the following scientific statements are true and which are false?
1) [ ] The formal charge of O in O2 molecule is -1
2) [ ] Covalent bonds are formed by sharing electrons between atoms.
3) [ ] Alkanes are water insoluble due to intra-molecular forces
4) [ ] Free radical addition is a characteristic reaction of saturated hydrocarbons
5) [ ] Free radical chlorination of propane is the ideal method to prepare 1-
chloropropane.
10) Please answer the following question in details
In light of your understanding to hybridization and orbitals shape and overlapping; draw a sketch for 1-bromo-3-chloropropene, to show each type of hybridization on the C atoms and how the δ and bond are formed in terms of orbitals overlapping
7 11) Low reactivity gives high selectivity. Comment on this statement with example
12) Please give the IUBAC names for the following simple compounds
I
Br
Cl
Cl F Cl
13) What is IUPAC stands for?
8