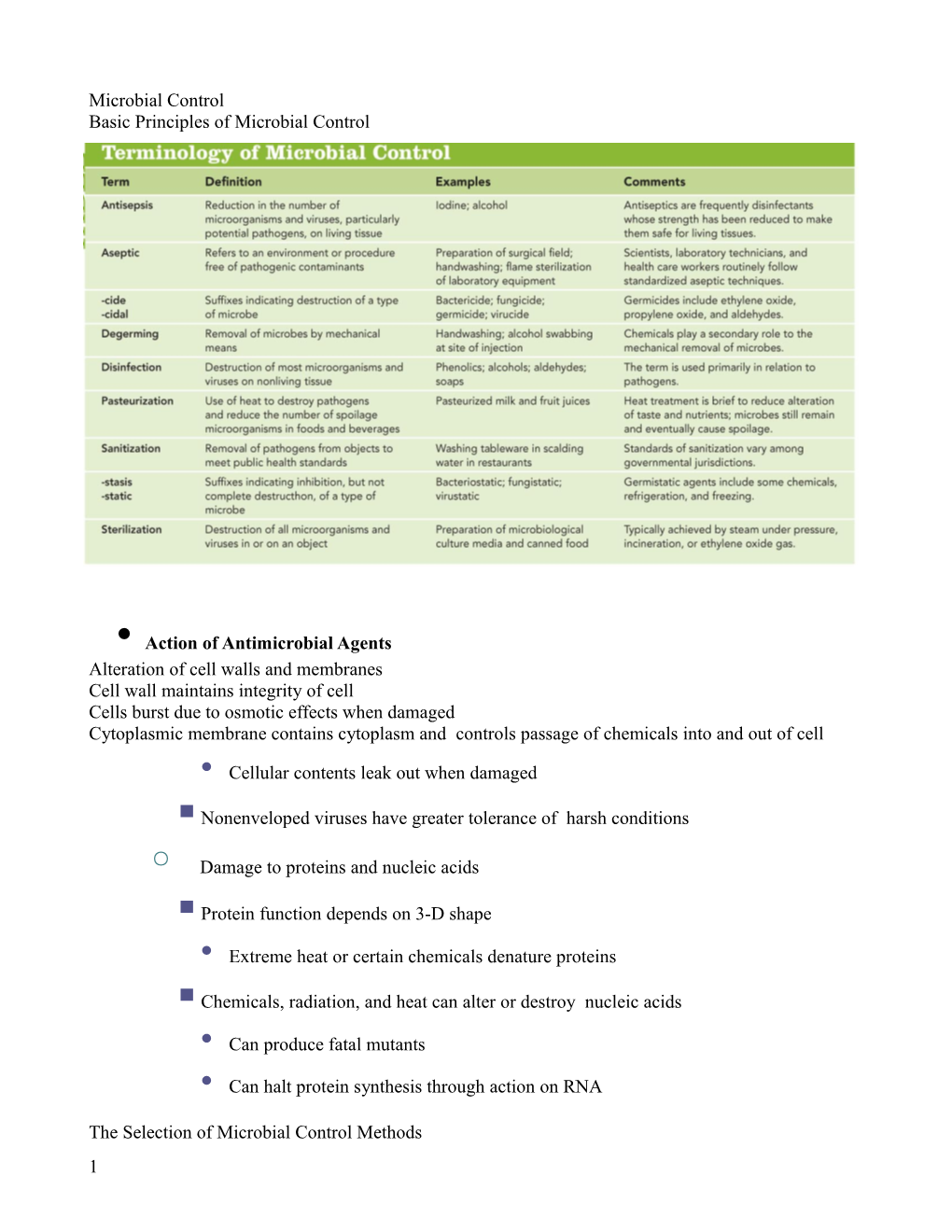
Microbial Control Basic Principles of Microbial Control
• Action of Antimicrobial Agents Alteration of cell walls and membranes Cell wall maintains integrity of cell Cells burst due to osmotic effects when damaged Cytoplasmic membrane contains cytoplasm and controls passage of chemicals into and out of cell • Cellular contents leak out when damaged
▪Nonenveloped viruses have greater tolerance of harsh conditions
◦ Damage to proteins and nucleic acids
▪Protein function depends on 3-D shape • Extreme heat or certain chemicals denature proteins
▪Chemicals, radiation, and heat can alter or destroy nucleic acids • Can produce fatal mutants • Can halt protein synthesis through action on RNA
The Selection of Microbial Control Methods 1 Ideally, agents should be:
• Inexpensive
• Fast-acting
• Stable during storage
• Capable of controlling microbial growth while being harmless to humans, animals, and objects
• Factors Affecting the Efficacy of Antimicrobial Methods
◦ Site to be treated Harsh chemicals and extreme heat cannot be used on humans, animals, and fragile objects Method of microbial control based on site of medical procedure
Relative susceptibilities of microbes to antimicrobial agents
◦ Factors Affecting the Efficacy of Antimicrobial Methods
◦ Relative susceptibility of microorganisms
▪Germicides classified as high, intermediate, or low effectiveness 2 • High-level kill all pathogens, including endospores • Intermediate-level kill fungal spores, protozoan cysts, viruses, and pathogenic bacteria • Low-level kill vegetative bacteria, fungi, protozoa, and some viruses
◦ Methods for Evaluating Disinfectants and Antiseptics ▪ Phenol coefficient • Evaluates efficacy of disinfectants and antiseptics by comparing an agent’s ability to control microbes to phenol • Greater than 1.0 indicates agent is more effective than phenol • Has been replaced by newer methods ◦ Methods for Evaluating Disinfectants and Antiseptics ▪ Use-dilution test • Metal cylinders dipped into broth cultures of bacteria • Contaminated cylinder immersed into dilution of disinfectant • Cylinders removed and placed into tube of medium to see how much bacteria survived • Most effective agents entirely prevent growth at highest dilution • Current standard test in the U.S. • New standard procedure being developed
▪ Kelsey-Sykes capacity test • Alternative assessment approved by the European Union • Bacterial suspensions added to the chemical being tested • Samples removed at predetermined times and incubated Lack of bacterial reproduction reveals minimum time required for the disinfectant to be effective
▪ In-use test • Swabs taken from objects before and after application of disinfectant or antiseptic • Swabs inoculated into growth medium and incubated • Medium monitored for growth • Accurate determination of proper strength and application procedure for each specific situation • • Physical Methods of Microbial Control ◦ Heat-Related Methods ▪ Effects of high temperatures • Denature proteins • Interfere with integrity of cytoplasmic membrane and cell wall • Disrupt structure and function of nucleic acids ▪ Thermal death point • Lowest temperature that kills all cells in broth in 10 min ▪ Thermal death time
3 • Time to sterilize volume of liquid at set temperature
▪ Moist heat • Used to disinfect (remove organisms and spores), sanitize (kill organisms but not necessarily their spores), and sterilize (kill all organisms and spores) • Denatures proteins and destroys cytoplasmic membranes • More effective than dry heat • Methods of microbial control using moist heat • Boiling • Autoclaving • Pasteurization • Ultrahigh-temperature sterilization
• Boiling • Kills vegetative cells of bacteria and fungi, protozoan trophozoites, and most viruses • Boiling time is critical ▪ Different elevations require different boiling times • Endospores, protozoan cysts, and some viruses can survive boiling
▪ Moist heat • Autoclaving • Pressure applied to boiling water prevents steam from escaping • Boiling temperature increases as pressure increases • Autoclave conditions – 121ºC, 15 psi, 15 min • Pasteurization • Used for milk, ice cream, yogurt, and fruit juices • Not sterilization ▪ Heat-tolerant microbes survive • Pasteurization of milk ▪ Batch method ▪ Flash pasteurization (High temp, short time) ▪ Ultrahigh-temperature pasteurization (very short time) ◦ ◦ Pasteurization of milk Batch method ◦ The batch method uses a vat pasteurizer which consists of a jacketed vat surrounded by either circulating water, steam or heating coils of water or steam. ◦ Pasteurization of milk Ultrahigh-temperature method • Heating for 1-2 seconds at a temperature exceeding 135°C (275°F), which is the temperature required to kill spores in milk. • The most common UHT product is milk, but the process is also used for fruit juices, cream, soy milk, yogurt, wine, soups, and stews. • Can cause browning and change the taste and smell of dairy products. • UHT milk has a typical shelf life of six to nine months, until opened ▪ Ultrahigh-temperature sterilization • 140ºC for 1 sec, then rapid cooling 4 • Treated liquids can be stored at room temperature
▪ Dry heat • Used for materials that cannot be sterilized with moist heat • Denatures proteins and oxidizes metabolic and structural chemicals • Requires higher temperatures for longer time than moist heat • Incineration is ultimate means of sterilization ◦ Refrigeration and Freezing ▪ Decrease microbial metabolism, growth, and reproduction • Chemical reactions occur slower at low temperatures • Liquid water not available ▪ Psychrophilic microbes can multiply in refrigerated foods ▪ Refrigeration halts growth of most pathogens ▪ Slow freezing more effective than quick freezing ▪ Organisms vary in susceptibility to freezing ◦ Dessication and Lyophilization ▪ Drying (98% of the water is removed) inhibits growth due to removal of water ▪ Lyophilization (freeze-drying) • Substance is rapidly frozen and sealed in a vacuum • Substance may also be turned into a powder ▪ Used for long-term preservation of microbial cultures • Prevents formation of damaging ice crystals
◦ Osmotic Pressure
▪ High concentrations of salt or sugar in foods to inhibit growth
▪ Cells in hypertonic solution of salt or sugar lose water
▪ Fungi have greater ability than bacteria to survive hypertonic environments
◦ Radiation
▪ Ionizing radiation
• Wavelengths shorter than 1 nm • Electron beams, gamma rays
• Ejects electrons from atoms to create ions
• Ions disrupt hydrogen bonding, cause oxidation, and create hydroxide ions
5 • Hydroxide ions denature other molecules (DNA)
• Electron beams – effective at killing but do not penetrate well
• Gamma rays – penetrate well but require hours to kill microbes
▪ Nonionizing radiation
• Wavelengths greater than 1 nm
• Excites electrons, causing them to make new covalent bonds • Affects 3-D structure of proteins and nucleic acids
• UV light causes pyrimidine dimers in DNA
• UV light does not penetrate well
• Suitable for disinfecting air, transparent fluids, and surfaces of objects
◦ Biosafety Levels
▪ Four levels of safety in labs dealing with pathogens
• Biosafety Level 1 (BSL-1) • Handling pathogens that do not cause disease in healthy humans
• Biosafety Level 2 (BSL-2) • Handling of moderately hazardous agents
• Biosafety Level 3 (BSL-3) • Handling of microbes in safety cabinets
• Biosafety Level 4 (BSL-4) • Handling of microbes that cause severe or fatal disease
• Chemical Methods of Microbial Control
6 • Affect microbes’ cell walls, cytoplasmic membranes, proteins, or DNA
• Effect varies with differing environmental conditions
• Often more effective against enveloped viruses and vegetative cells of bacteria, fungi, and protozoa
• Phenol and Phenolics
◦ Intermediate- to low-level disinfectants
◦ Denature proteins and disrupt cell membranes
◦ Effective in presence of organic matter
◦ Remain active for prolonged time
◦ Commonly used in health care settings, labs, and homes
◦ Have disagreeable odor and possible side effects •
• Alcohols
◦ Intermediate-level disinfectants
◦ Denature proteins and disrupt cytoplasmic membranes
◦ More effective than soap in removing bacteria from hands
◦ Swabbing of skin with 70% ethanol prior to injection
• Halogens
◦ Intermediate-level antimicrobial chemicals
◦ Believed to damage enzymes via oxidation or by denaturation
◦ Widely used in numerous applications
7 ▪Iodine tablets, iodophores, chlorine treatment, bleach, chloramines, and bromine disinfection
• Oxidizing Agents
◦ Peroxides, ozone, and peracetic acid
◦ Kill by oxidation of microbial enzymes
◦ High-level disinfectants and antiseptics
◦ Hydrogen peroxide (H2O2) can disinfect and sterilize surfaces
▪Not useful for treating open wounds due to catalase activity: the tissues convert it into H20 and 0ygen bubbles.
◦ Ozone treatment of drinking water
◦ Peracetic acid is an effective sporocide used to sterilize equipment
• Surfactants
◦ “Surface active” chemicals
▪Reduce surface tension of solvents
◦ Soaps and detergents
▪Soaps have hydrophilic and hydrophobic ends ▪ Good degerming agents but not antimicrobial
▪Detergents are positively charged organic surfactants
◦ Quats (Quaternary ammonium cations)
▪Low-level disinfectants; disrupts cell membranes
▪Ideal for many medical and industrial application
▪Good against fungi, amoeba, and enveloped viruses, but not endospores, Mycobacterium tuberculosis and non-enveloped viruses.
8 • Heavy Metals
◦ Heavy-metal ions denature proteins
◦ Low-level bacteriostatic and fungistatic agents
◦ 1% silver nitrate to prevent blindness caused by N. gonorrhoeae
◦ Thimerosal used to preserve vaccines
◦ Copper inhibits algal growth
• Aldehydes
◦ Compounds containing terminal –CHO groups
◦ Cross-link functional groups to denature proteins and inactivate nucleic acids
◦ Glutaraldehyde disinfects and sterilizes
◦ Formalin used in embalming and disinfection of rooms and instruments • •
• Gaseous Agents
◦ Microbicidal and sporicidal gases used in closed chambers to sterilize items
◦ Denature proteins and DNA by cross-linking functional groups
◦ Used in hospitals and dental offices
◦ Disadvantages
▪Can be hazardous to people
▪Often highly explosive
▪Extremely poisonous
▪Potentially carcinogenic
9 • Enzymes
◦ Antimicrobial enzymes act against microorganisms
◦ Human tears contain lysozyme
▪Digests peptidoglycan cell wall of bacteria
◦ Enzymes to control microbes in the environment
▪Lysozyme used to reduce the number of bacteria in cheese
▪Prionzyme can remove prions on medical instruments
• Antimicrobials
◦ Antibiotics, semi-synthetic, and synthetic chemicals
◦ Typically used for treatment of disease
◦ Some used for antimicrobial control outside the body
• Development of Resistant Microbes
◦ Little evidence that products containing antiseptic and disinfecting chemicals is beneficial to human or animal health
◦ Use of such products promotes development of resistant microbes
Antimicrobial Agents
• Chemicals that affect physiology in any manner
• Chemotherapeutic agents
◦ Drugs that act against diseases
◦ Drugs that treat infections
The History of Antimicrobial Agents
10 ◦ Semi-synthetics
▪ Chemically altered antibiotics that are more effective than naturally occurring ones
◦ Synthetics
▪ Antimicrobials that are completely synthesized in a la
Mechanisms of Antimicrobial Action
◦ Key is selective toxicity
◦ Antibacterial drugs constitute largest number and diversity of antimicrobial agents
◦ Fewer drugs to treat eukaryotic infections (protozoa, fungi, helminthes)
◦ Even fewer antiviral drugs
Inhibition of Cell Wall Synthesis
◦ Inhibition of bacterial wall synthesis
▪Most common agents prevent cross-linkage of NAM subunits
▪Beta-lactams are most prominent in this group ▪ Functional groups are beta-lactam rings ▪ Beta-lactams bind to enzymes that cross-link NAM subunits
▪Bacteria have weakened cell walls and eventually lyse
◦ Inhibition of synthesis of bacterial walls
▪Semi-synthetic derivatives of beta-lactams ▪ More stable in acidic environments
11 ▪ More readily absorbed ▪ Less susceptible to deactivation ▪ More active against more types of bacteria
▪Simplest beta-lactams – effective only against aerobic Gram-negatives
▪Vancomycin and cycloserine ▪ Interfere with particular bridges that link NAM subunits in many Gram-positives
▪Bacitracin ▪ Blocks secretion of NAG and NAM from cytoplasm ▪ Effective against Gram positives
▪Isoniazid and ethambutol ▪ Disrupt mycolic acid formation in mycobacterial species
▪Prevent bacteria from increasing amount of peptidoglycan
▪Have no effect on existing peptidoglycan layer
▪Effective only for growing cells
• Inhibition of Protein Synthesis
◦ Prokaryotic ribosomes are 70S (30S and 50S)
◦ Eukaryotic ribosomes are 80S (40S and 60S)
◦ Drugs can selectively target translation
◦ Mitochondria of animals and humans contain 70S ribosomes
▪Can be harmful
◦ Aminoglycosides: excellent against Gram negatives, partially effective against Gram positives
12 ▪amikacin (Amikin®)
▪gentamicin (Garamycin®)
▪kanamycin (Kantrex®)
▪neomycin (Mycifradin®)
▪streptomycin
▪tobramycin (TOBI Solution®, TobraDex®)
◦ Disruption of Cytoplasmic Membranes
▪ Some drugs form channel through cytoplasmic membrane and damage its integrity
▪ Amphotericin B attaches to ergosterol in fungal membranes
• Humans somewhat susceptible because cholesterol similar to ergosterol
• Bacteria lack sterols; not susceptible
▪ Azoles and allyamines inhibit ergosterol synthesis
▪ Polymyxin disrupts cytoplasmic membranes of Gram-negatives
• Oral form is toxic to human kidneys, so only used topically
▪ Some parasitic drugs act against cytoplasmic membranes
Which topical ointment is best? • Neomycin is an aminoglycoside antibiotic (disrupts protein synthesis). It has excellent activity against Gram-negative bacteria, and has partial activity against Gram-positive bacteria.
• Polymixin disrupts bacterial cell membranes by interacting with its phospholipids. They are selectively toxic for Gram-negative bacteria. • Bacitracin disrupts cell wall synthesis. Its action is on Gram-positive organisms. It can cause contact dermatitis and cross-reacts with allergic sensitivity to sulfa-drugs.
13 Which topical ointment is best: Neomycin or Triple Antibiotic (contains all three) ◦ Mechanisms of Antimicrobial Action
Inhibition of Metabolic Pathways ◦ Antimetabolic agents can be effective when pathogen and host metabolic processes differ ◦ Quinolones interfere with the metabolism of malaria parasites ◦ Heavy metals inactivate enzymes ◦ Some agents disrupt glucose uptake by many protozoa and parasitic worms ◦ Some drugs block activation of viruses ◦ Antiviral agents can target unique aspects of viral metabolism ▪ Amantadine, rimantadine, and weak organic bases prevent viral uncoating ◦ Protease inhibitors interfere with an enzyme that HIV needs in its replication cycle
Inhibition of Nucleic Acid Synthesis ◦ Several drugs block DNA replication or mRNA transcription ◦ Drugs often affect both eukaryotic and prokaryotic cells ◦ Not normally used to treat infections ◦ Used in research and perhaps to slow cancer cell replication ◦ Nucleotide analogs ▪ Interfere with function of nucleic acids ▪ Distort shapes of nucleic acid molecules and prevent further replication, transcription, or translation ▪ Most often used against viruses ▪ Effective against rapidly dividing cancer cells
Acyclovir • Acyclovir is used to decrease pain and speed the healing of herpes sores or blisters in people who have varicella (chickenpox), herpes zoster (shingles; a rash that can occur in people who have had chickenpox in the past), and first-time or repeat outbreaks of genital herpes (a herpes virus infection that causes sores to form around the genitals and rectum from time to time). • Acyclovir is also sometimes used to prevent outbreaks of herpes sores in people who are infected with the virus. • Acyclovir disrupts nucleic acid function. It works by stopping the spread of the herpes virus in the body. Acyclovir will not cure herpes or protect others from catching it.
◦ Quinolones and fluoroquinolones ▪ Act against prokaryotic DNA gyrase (enzyme that is needed for DNA to unwind during replication) ◦ Inhibitors of RNA polymerase (enzyme used during transcription) ◦ Reverse transcriptase inhibitors ▪ Act against an enzyme HIV uses in its replication cycle ▪ Does not harm people because humans lack reverse transcriptase
14 Prevention of Virus Attachment ◦ Attachment antagonists block viral attachment or receptor proteins ◦ New area of antimicrobial drug development
Clinical Considerations in Prescribing Antimicrobial Drugs
Ideal Antimicrobial Agent ◦ Readily available ◦ Inexpensive ◦ Chemically stable ◦ Easily administered ◦ Nontoxic and nonallergenic ◦ Selectively toxic against wide range of pathogens
Spectrum of Action ▪ Number of different pathogens a drug acts against • Narrow-spectrum effective against few organisms • Broad-spectrum effective against many organisms • May allow for secondary or superinfections to develop • Killing of normal flora reduces microbial antagonism
Spectrum of action for selected antimicrobial agents
• • • • • • •
15 • Efficacy ◦ Ascertained by ▪ Diffusion susceptibility test ▪ Minimum inhibitory concentration test ▪ Minimum bactericidal concentration test
• Routes of Administration ◦ Topical application of drug for external infections ◦ Oral route requires no needles and is self-administered ◦ Intramuscular administration delivers drug via needle into muscle ◦ Intravenous administration delivers drug directly to bloodstream ◦ Must know how antimicrobial agent will be distributed to infected tissues
• Safety and Side Effects ◦ Toxicity ▪ Cause of many adverse reactions poorly understood ▪ Drugs may be toxic to kidneys, liver, or nerves ▪ Consideration needed when prescribing drugs to pregnant women ◦ Allergies ▪ Allergic reactions are rare but may be life threatening ▪ Anaphylactic shock
◦ Disruption of normal microbiota ▪ May result in secondary infections ▪ Overgrowth of normal flora causing superinfections ▪ Of greatest concern for hospitalized patients
Resistance to Antimicrobial Drugs The Development of Resistance in Populations ▪ Some pathogens are naturally resistant ▪ Resistance by bacteria acquired in two ways • New mutations of chromosomal genes • Acquisition of resistance genes (R-plasmids) via transformation, transduction, and conjugation
◦ Mechanisms of Resistance ▪ At least six mechanisms of microbial resistance • Production of enzyme that destroys or deactivates drug • Slow or prevent entry of drug into the cell • Alter target of drug so it binds less effectively • Alter their metabolic chemistry • Pump antimicrobial drug out of the cell before it can act • Mycobacterium tuberculosis produces MfpA protein
16 • Binds DNA gyrase preventing the binding of fluoroquinolone drugs ◦ Multiple Resistance and Cross Resistance ▪ Pathogen can acquire resistance to more than one drug ▪ Common when R-plasmids exchanged ▪ Develop in hospitals and nursing homes • Constant use of drugs eliminates sensitive cells ▪ Superbugs ▪ Cross resistance ◦ Retarding Resistance ▪ Maintain high concentration of drug in patient for sufficient time • Kills all sensitive cells and inhibits others so immune system can destroy ▪ Use antimicrobial agents in combination • Synergism vs. antagonism ▪ Use antimicrobials only when necessary ▪ Develop new variations of existing drugs • Second-generation drugs • Third-generation drugs ▪ Search for new antibiotics, semi-synthetics, and synthetics • Bacteriocins • Design drugs complementary to the shape of microbial proteins to inhibit them Vaccination • Vaccine – use the immune system to protect against infectious disease Types of vaccines • attenuated (weakened) microbe; virulence factors are removed • heat-killed / chemically killed microbe • toxoids • Passive versus Adaptive vaccination • passive – immune system products from another • mothers milk (presence of IgA) • gamma-globulin (anti-bee venom, anti-hepatitis A, etc) • active – stimulate individuals immune system to produce memory cells
17