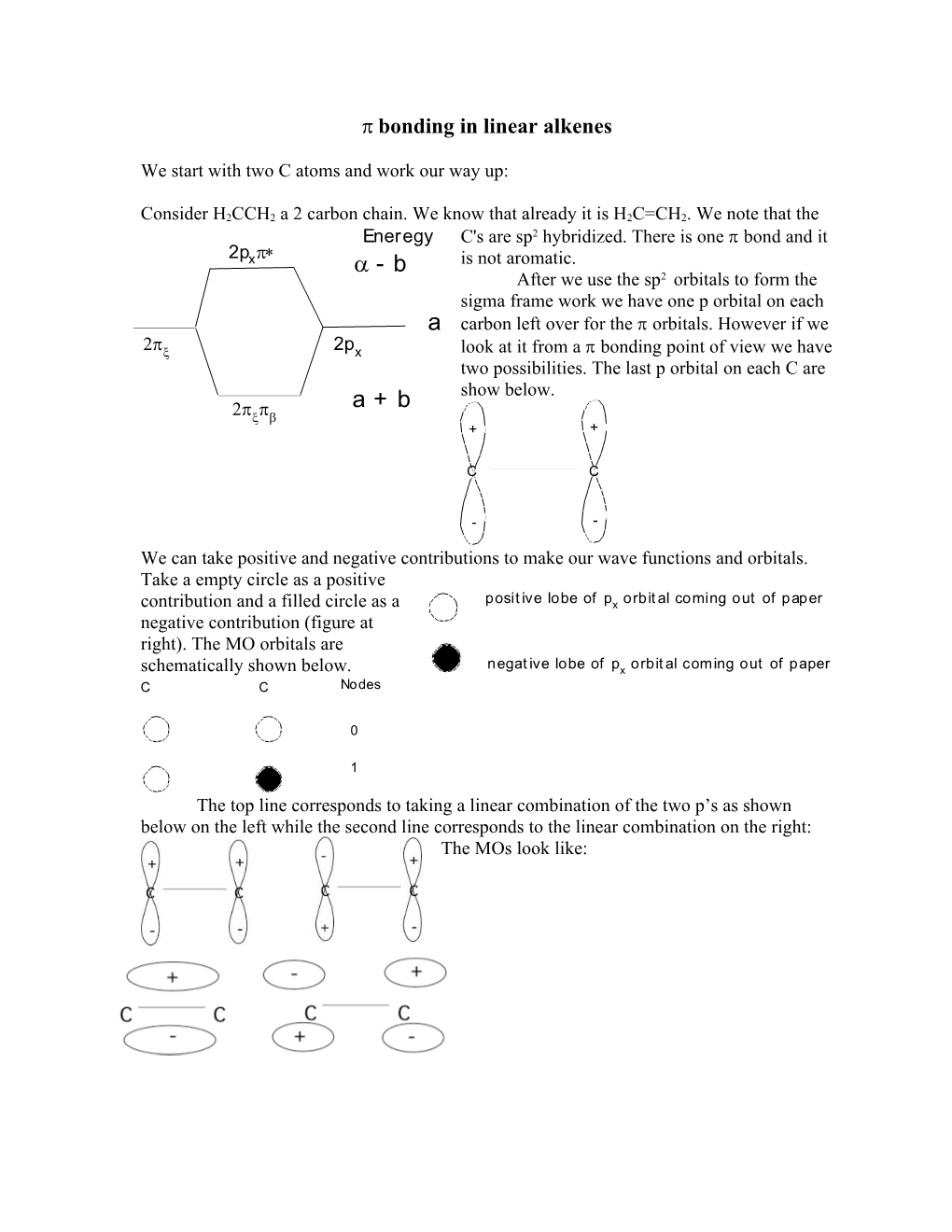
bonding in linear alkenes
We start with two C atoms and work our way up:
Consider H2CCH2 a 2 carbon chain. We know that already it is H2C=CH2. We note that the Eneregy C's are sp2 hybridized. There is one bond and it 2p xp* - b is not aromatic. a 2 After we use the sp orbitals to form the sigma frame work we have one p orbital on each a carbon left over for the orbitals. However if we 2px 2px look at it from a bonding point of view we have two possibilities. The last p orbital on each C are a + b show below. 2pxpb + +
C C
- -
We can take positive and negative contributions to make our wave functions and orbitals. Take a empty circle as a positive contribution and a filled circle as a posit ive lobe of px orbit al coming out of paper negative contribution (figure at right). The MO orbitals are schematically shown below. negat ive lobe of px orbit al coming out of paper C C Nodes
0
1
The top line corresponds to taking a linear combination of the two p’s as shown below on the left while the second line corresponds to the linear combination on the right: The MOs look like: Or to make it simpler use + and – signs for the contribution of the AO's Nodes C C + + 0 + - 1 Is is written with the number of nodes going down so remember that if you want to write the orbitals in terms of energy you need to write then in the opposite direction: C C Nodes + - 1 a -b + + 0 a + b Where is the energy of the AOs and is the amount that the bond stabilizes or destabilizes the MO. Note that we can not do three carbons stable molecule since H2CCHCH2 is a radical and the last C does not have a full octet! Draw the Lewis dot structure and you will see. It 3 2 will react to form H2CCHCH3 and now the last carbon is sp and not sp . However, you can still draw the radical try it. 2 Now we go on to four carbons H6C4 where again all C's are sp hybridized: H H C C H
H C C H H Nodes C C C C + - + - 3 a + 1 .6 b + - - + 2 a + 0 .6 b + + - - 1 a - 0 .6 b + + + + 0 a - 1 .6 b You note that if you draw the Lewis dot structure you get two double bonds and one single bond between each C. Remember this is only the orbitals we are talking about and the orbitals already have a bond between each C atom. In the MO bonding scheme above there is one electron in each of the p orbitals that you use to form the bonds or 4 electrons. With 4 electrons you fill up the two lowest orbitals. Both of these levels have a bond between the two C atoms on the left side and the two on the right. The lowest level also has a bond between the two central C atoms. The second orbital has bonding between the first and second C and a nodebetween the second and third. The net result is that for the 2 filled MO’s we get a lot of extra bonding between the end C’s and only a little extra between the center two. In the molecule, the bond lengths between the two end carbons is a little more then that of a C=C double bond and between the center two carbons is a little shorter that a single bond. Look at the pluses and minuses in the table above as a matrix. You see that: The first column and the bottom row are the same. So is the second column and the second row, etc. It is symmetric along a line from the low left to the upper right.
So what are the rules for drawing the MO picture of the bonds:
A) Keep the rows with odd number of nodes symmetric to inversion (+ - | - +)) B) Keep rows with even number of nodes antisymmetric to inversion (+ - |+- -) C) Column i is the same as the reverse of the row with nodes i-1 (first column (all +) is same as bottom row (all +) , second column (- - + +) is same as second row(+ + - -)
Procedure: 1. Start at the bottom and work up. 2. Write the zero node row as all + on the bottom. 3. Write the first column as all + 4. Write the node 1 row above the node zero row as half + and half – 5. Copy this to the second column 6. Use first part of row with 2 nodes to fill in end of row (remember it is symmetric) 7. Fill in rest of row with 2 nodes to make 2 nodes 8. etc
Ok now try do an 8 carbon chain: Nodes C C C C C C C C MO’s + - + - + - + - 7 a - 1 .9 b + - + - - + - + 6 a - 1 .5 b + - + - + - 5 a - 1 .0 b + - - + + - - + 4 a - 0 .3 b + + - - + + - - 3 a + 0 .3 6 b + + - - + + 2 a + 1 .0 b + + + + - - - - 1 a + 1 .5 b + + + + + + + + 0 a + 1 .9 b There are other ways to write it also that follows the rules. At this level it does C C C C C C C C Nodes not matters since you + - + - + - + - 7 cannot tell them apart. The two ways of + - + - - + - + 6 writing the 2 node + - + + - - + - 5 orbitals actually are + - - + + - - + 4 not exactly correct and a linear combination of + + - - + + - - 3 the two ways is needed + + + - - + + + 2 but don't worry about + + + + - - - - 1 that. + + + + + + + + 0
Energies of the levels for the linear Hn+2Cn hydrocarbons: are listed below for information note that all levels are single levels and are not degenerate: n lowest center Highest level level 2 3 4 5 8 10 20 Note that as n gets bigger the lowest and highest orbitals are closer and closer to and that the spacing between orbitals becomes smaller. This is the beginning of the band structure that is seen in semiconductors and metals. I have put in the odd numbered length molecules but these are not stable to reacting and adding another CH bond. However you can talk about the radical and so they have been added. You note that for even number of C atoms you do not get any orbital that is in the center. For all the compounds the orbitals have an even spacing in energy. bonding in Cyclic Alkenes Now we try cycles, the nodes are shown in red below here are the rules: 1. Draw the outline of the compound with one atom down. 2. Put a line at the level of every vertex or C atom in the compound, these are the MOs. 3. Make a table showing the contributions to each MO as above. 4. The orbitals lower than the center are bonding orbitals and the ones higher are antibonding orbitals Remember that for an odd number the molecule is a radical (i.e. one C does not have a full octet).
Here is H6C3
C — C — C nodes Energy t ype + - + 1 a - b p* + - 1 a - b p* 3 1 + + + 0 a + 2 b p 2 The two end carbons in the diagram are attached to each other so the two top orbitals both have the same number of nodes. H6C3 has one unpaired electron, it is a radical and the last electron is in an antibonding orbital. Thus it only has about a half of a bond distributed over three bonds. The highest two orbitals are degenerate (they have the same energy). This for the molecule as drawn. If it distorts in some way it can break the degeneracy between those bond. This will stabilize the molecule since the electron can go in the low energy orbital. The distortion will involve one of the C-C bonds becoming a double bond and the other two being single bonds. This sort of distortion is important later.
Here is H4C4
C — C — C —C nodes + - + - 2 a - 1 b + - - + 1 a + + - - 1 + + + + 0 a + 2 b 1
This is a true molecule and not a radical. It has a bonding and an antibonding orbital and two nonbonding orbitals. There are two electrons in the bonding orbital and two in the nonbonding orbitals. Thus it really has only one extra bond and so it is not any better (actually worse) then the Lewis structure that has two double bonds and two single bonds. Again since the two nonbonding orbitals are degenerate the molecule will distort. The two electrons will go into the stable orbital and pair. Thus this molecule H H distorts and it will not be a square more likely a rectangle with two long C-C single bonds and two short C-C double bonds, see figure at C C right. The bonds are localized and are not spread out over the whole molecule. It is not aromatic. C C
H5C5. H H
C — C — C — C — C nodes + - + - + 2 a- 1 .6 b p* + - + - 2 a- 1 .6 b p* + - - - + 1 a +0 .6 b p + + - - 1 a +0 .6 b p 1 + + + + + 0 a + 2 b p Here again we have a radical when it is netural. The bonding has three bonding and two antibonding orbitals. For the neutral we have five electrons in the bonding orbitals so that is pretty good, however the partially filled bonding orbitals are degenerate and so the molecule will probably distort to split that degeneracy and put two electrons in the lower orbital and one in the higher orbital. It will also react to form H6C5, which is not a radical H
H H C H C C C C H – and is shown below. H The anion, H5C5 , has an extra electron and now there are 6 bonding electrons and so it is stable, planar and aromatic. + Consider the cation H5C5 , the positive ion, 2 electrons are in the degenerate bonding pair and so it is again not stable. It will distort to form two double bonds and three single bonds. Here is benzene, H6C6. It has 6 p orbitals and 6 electrons and they fill up the three bonding orbitals. Thus it is planar and aromatic. 6 5 1
4 2 C — C — C — C — C — C nodes 3 + + + + + + 0 + + + - - - 1 + - - + 1 + - + - 2 + - + + - + 2 + - + - + - 3
C — C — C — C — C — C — C — C nodes + + + + + + + + 0 + + + + - - - - 1 + + - - - - + + 1 + + - - + + - - 2 - + + - - + + - 2 + - + - + - 3 + - + - + - 3 + - + - + - + - 4 1 2
H8C8, has 8 p orbitals and 8 electrons. They fill up the three lowest level and the last two electrons go into the nonbonding degenerate pair. Again we have a situation that there is an unfilled degenerate orbital and it will want to distort. The molecule will distort to have four double bonds and four single bonds, shown to the right. Energy levels for cycle hydrocarbons HnCn note that most levels other than the first and last are doubly degenerate. The last level for n odd is also degenerate n lowest Middle Highest level Level level 3 4 5 1 6 … … 7 0 … … 1 8 4 . . 20 … 1. Note that the lowest energy orbitals are always at +This is the limit for n large in straight chain HC's. If we look at a cyclic hydrocarbon as a straight chain hydrocarbon that has a bond from the last C to the first (physicists call this cyclic boundary conditions) then we see that once you do this the lowest energy orbital is at the energy for a straight chain hydrocarbon that has a very long chain. 2. All the odd numbered carbon cyclics have a double degenerate highest orbital and no nonbonding orbital. 3. All the even number orbitals that have 4n carbons (4,8,12,16,…) have a nonbonding orbital that is partially filled. This means that they are not aromatic. 4. All the even number orbitals that have 4n+2 carbons (6,10,14,..) have no nonbonding orbirtal and have half of their orbitals as bonding and half as nonbonding. Since they have the same number of electrons as orbitals half of the orbitals are filled and half are empty (remember that orbitals take 2 electrons). Thus these cyclic compounds have filled boning orbitals and like to be planar and are aromatic.