Classification of Minerals
Classified on the basis of anionic groups Minerals in each group have grossly similar crystallographic properties and tend to occur in the same geological settings / environments - silicates – igneous and metamorphic rocks - carbonates – sedimentary rocks (also some igneous) - sulfides – hydrothermal systems (fluid–rock dominated)
Mineral Classes
1) Native Elements 2) Sulfides + Sulfosalts (As, Sb) S2- 3) Oxides O2- 4) Hydroxides OH- 5) Halides Cl-, F-, Br- 6) 2- Carbonates CO3 7) - Nitrates NO3 8) Borates BxOy(OH)z 9) 5- Phosphates PO4 10) 2- Sulfates SO4 11) 2- 2- Tungstates-Molybdates WO4 MoO4 12) 4- Silicates SiO4
Mineral Class Family Group Species Varieties
Silicates
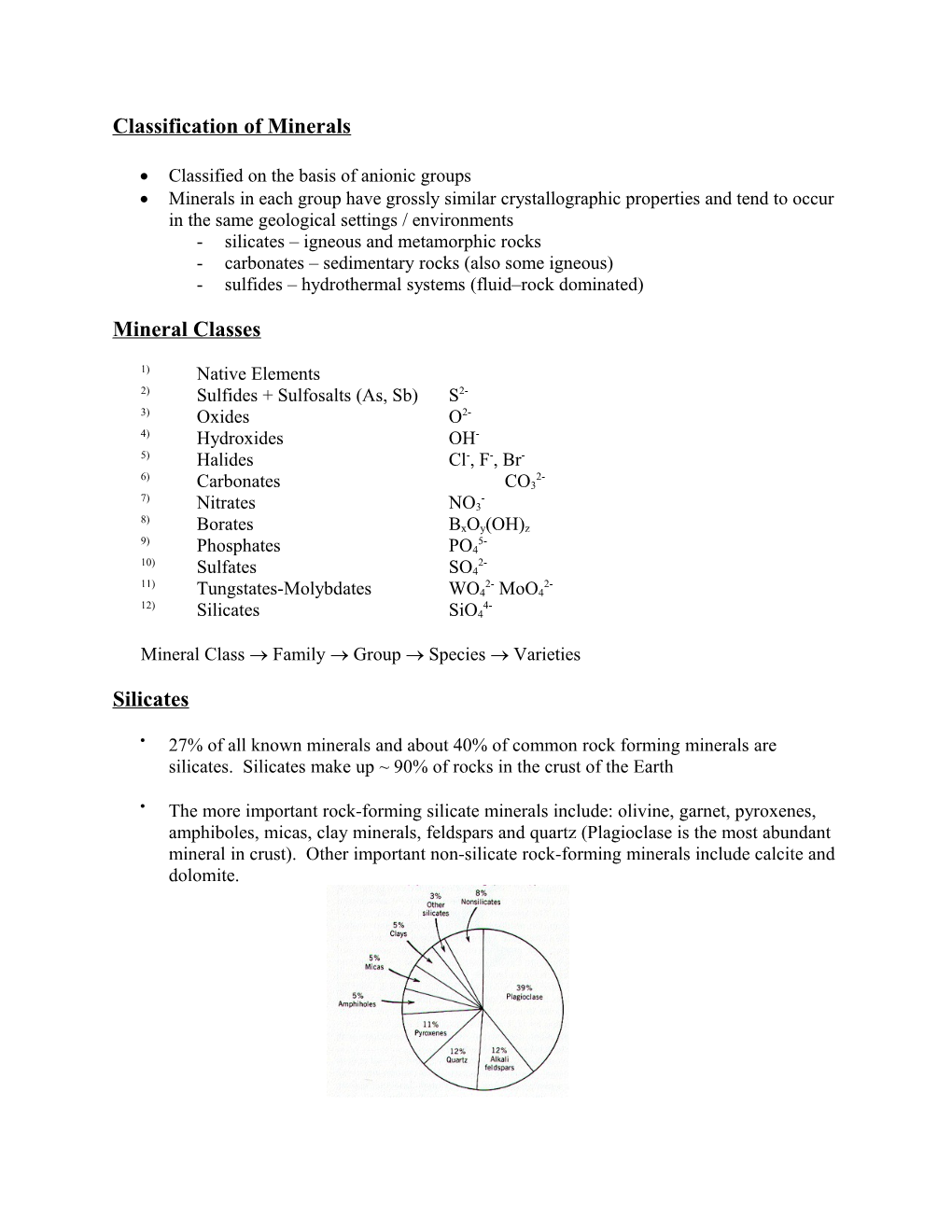
27% of all known minerals and about 40% of common rock forming minerals are silicates. Silicates make up ~ 90% of rocks in the crust of the Earth
The more important rock-forming silicate minerals include: olivine, garnet, pyroxenes, amphiboles, micas, clay minerals, feldspars and quartz (Plagioclase is the most abundant mineral in crust). Other important non-silicate rock-forming minerals include calcite and dolomite.
Important in Igneous and Metamorphic Rocks and sediments derived from them
Important elements are: Si 4+; Al3+; Fe2+; Fe3+; Mg2+; Ca2+; Na+; K+; Ti4+; Cr3+; Mn2+
Radius of Si 4+:O2- 0.26 Å tetrahedral co-ordinated
Si-O bond is ~ 50% ionic and ~50% covalent
SiO4 has a net – 4 charge such that each oxygen has a potential to bond to another Si, thereby forming links between SiO4 tetrahedra
Silicates are classified on the basis of how the silica tetrahedral are linked
Al3+ is very important in silicates because it is the 2nd most abundant metal and the 3rd most abundant element.
Ionic Radius of Al3+ = 0.39 Å and radius ratio of Al3+:O2- 0.286
Al3+ substitutes for Si4+ in tetrahedral sites
Because radius ratio is close to octahedral co-ordination, Al3+ can also go into the 6 co- ordinated site (octahedral)
Substitution of Al3+ into tetrahedral sites allows additional elements Mg2+; Fe2+; Fe3+; Mn2+; Cr3+; Ti4+ to be put in octahedral sites (6-fold co-ordination)
Ca2+ and Na+ go into 8-fold (cubic) sites and K+; Rb+ and Ba2+ go into 8 to 12 co-ordinated sites General Formula for Silicate Minerals
Xm Yn (ZpOq) Wr (All formulas must be charge balanced)
8-Fold 6-Fold 4-Fold OH-, Cl-, F-
(1) -4 Nesosilicates (SiO4)
- independent tetrahedral linked by octahedral sites - O:Si = 4:1
(2) -6 Sorosilicates (Si2O7)
- two tetrahedral linked by a single oxygen atom - O:Si = 3.5:1
(3) -12 -6 Cyclosilicates (Si6O18) or (Si3O9)
- rings - O:Si = 3:1 (4) -2 Inosilicate (a) Single Chain (SiO3)
- O:Si = 3:1
-6 (b) Double Chain (Si4O11)
- O:Si = 2.75:1 (5) -2 Phyllosilicates (Si2O5) (sheet silicates)
- rings of tetrahedral linked together - O:Si = 2.5:1
(6) 0 Tectosilicates (SiO2) (3-D Framework)
- O:Si = 2:1