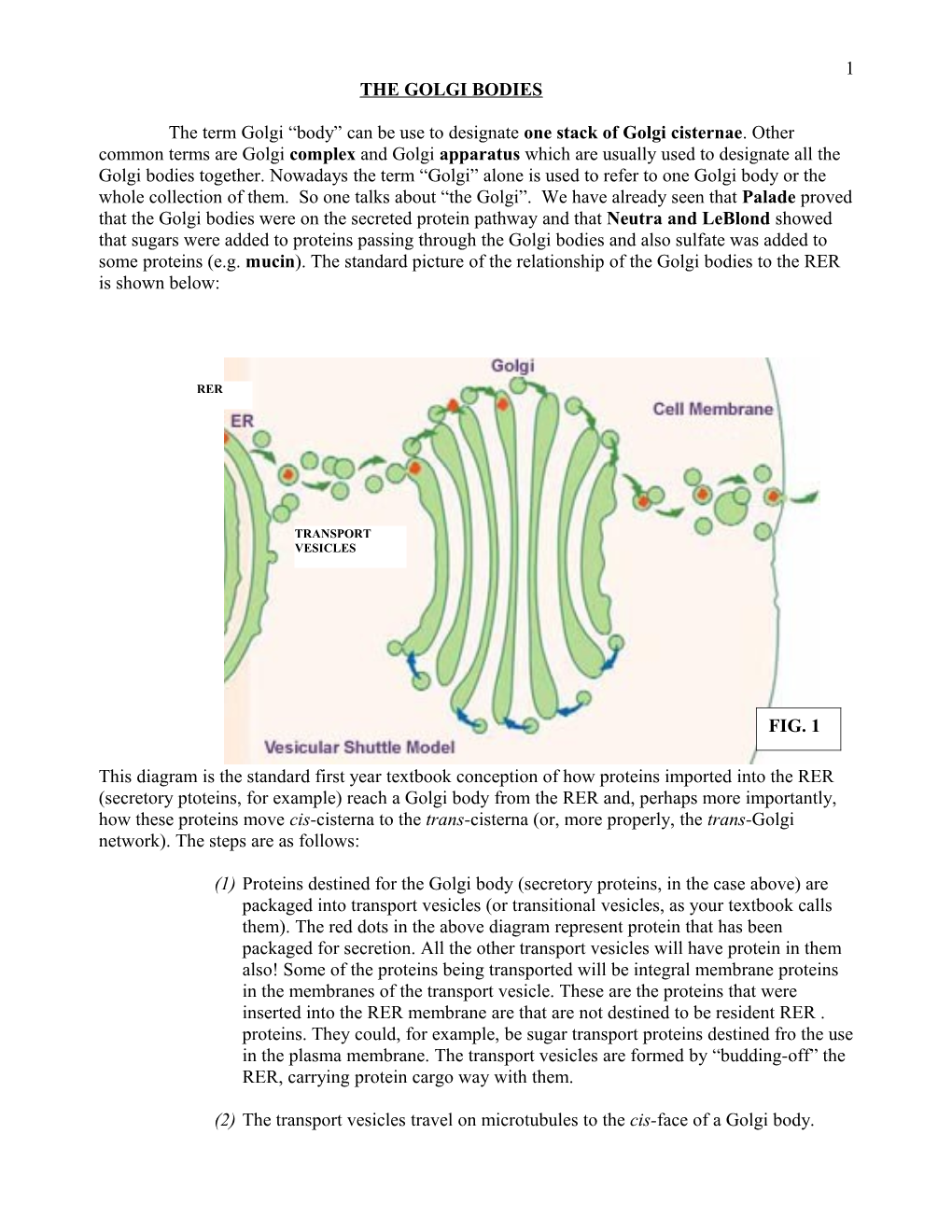
1 THE GOLGI BODIES
The term Golgi “body” can be use to designate one stack of Golgi cisternae. Other common terms are Golgi complex and Golgi apparatus which are usually used to designate all the Golgi bodies together. Nowadays the term “Golgi” alone is used to refer to one Golgi body or the whole collection of them. So one talks about “the Golgi”. We have already seen that Palade proved that the Golgi bodies were on the secreted protein pathway and that Neutra and LeBlond showed that sugars were added to proteins passing through the Golgi bodies and also sulfate was added to some proteins (e.g. mucin). The standard picture of the relationship of the Golgi bodies to the RER is shown below:
RER
TRANSPORT VESICLES
FIG. 1
This diagram is the standard first year textbook conception of how proteins imported into the RER (secretory ptoteins, for example) reach a Golgi body from the RER and, perhaps more importantly, how these proteins move cis-cisterna to the trans-cisterna (or, more properly, the trans-Golgi network). The steps are as follows:
(1) Proteins destined for the Golgi body (secretory proteins, in the case above) are packaged into transport vesicles (or transitional vesicles, as your textbook calls them). The red dots in the above diagram represent protein that has been packaged for secretion. All the other transport vesicles will have protein in them also! Some of the proteins being transported will be integral membrane proteins in the membranes of the transport vesicle. These are the proteins that were inserted into the RER membrane are that are not destined to be resident RER . proteins. They could, for example, be sugar transport proteins destined fro the use in the plasma membrane. The transport vesicles are formed by “budding-off” the RER, carrying protein cargo way with them.
(2) The transport vesicles travel on microtubules to the cis-face of a Golgi body. 2 (3) The transport vesicles fuse with the cis-cisterna of the Golgi body.
(4) The transport vesicle membrane, along with its cargo integral proteins, becomes a temporary part of the cisternal membrane.
(5) The cargo proteins in the lumen of the transport vesicles ares now in the cisternal lumen.
(6) In the cis-cisterna resident enzymes, characteristic of the cis-cisterna, modify the newly imported proteins e.g. by adding sugars. This glycosylation is done by glycosyltranseferases. Each cisterna has its own unique set of these enzymes, and others.
(7) Once the proteins have been modified by the enzymes of the cis-cisterna, they must be moved on to the next cisterna. This is done, once again, by the budding- off of transport vesicles.
(8) And the same procedure is repeated at all the cisterna of the Golgi body, until the trans-Golgi cisterna (or trans-Golgi network). See Fig. 1 above.
(9) What would the retrograde COPI transport vesicles be doing in the in the vesicular shuttle model? Presumably they are recycling membrane back to the cis-cisterna so that anterograde COPII transport vesicles can continue to be made.
The transport vesicles are coated vesicles. The peroxisomes, for example, do not have coats. The coat of the transport vesicles consists mainly of so-called coatamer proteins (COPs). [coatamer = coat + mer = part]. The transport vesicles nowadays are more usually called COPII vesicles. There are also COPI vesicles, but they move in a retrograde direction (more about them later).
There is a general consensus that at least most or the proteins move from the RER to the Golgi bodies in transport vesicles (or COPI vesicles). But as we will see there is not a consensus that the proteins move from cisterna to cisterna by transport vesicles. But more about that later.
Let us look at a transport vesicle coat. By doing this we will also see something else about such vesicles, and others such as synaptic vesicles and secretory vesicles i.e. how are they “captured” by their targets?
LUMEN OF RER RECEPTOR PROTEIN FOR THE GTP- CYTOSOL CYTOSOL BINDING PROTEIN 3
GTP BINDING PROTEIN
COATOMER PROTEIN
RESIDENT INTEGRAL MEMBRANE PROTEINS
CARGO-BINDING PROTEINS
SOLUBLE CARGO PROTEINS GTP BINDING PROTEIN (THE BOUND GTP IS NOT SHOWN)
INTEGRAL MEMBRANE PROTEIN V-SNARE CARGO
A TRANSPORT VESICLE (ASLO KNOWN AS A COPII VESICLE)
Pi UNCOATING CAUSED BY HYDROLYSIS OF THE GTP IN THE GTP-BINDING PROTEIN
FIG. 2
GTP BINDING PROTEIN This is a re-labelled (me) diagram of COATOMER PROTEIN a diagram from Lodish et al.
There are some things specific to the discussion of the transport vesicles (COPII vesicles) and some other things that are of very general significance in cell biology. This is one reason I am covering this. Consider the following points:
(1) There are both soluble (in the lumen) and integral membrane proteins that must remain as resident proteins in the RER. Their residence in the RER is eesential to the proper functioning of the RER. (What are some of these proteins?) These proteins must not become “cargo” in the transport vesicles.
(2) The proteins that are destined for travel to the Golgi bodies must be concentrated in some areas for packaging into the transport vesicles (COPII vesicles).
(3) The formation of a coat on the vesicle serves two functions: 4
- it serves as a “marshalling area” for cargo proteins that are to be transported
- it causes a curvature in the membrane to allow the “budding-off” of the transport vesicle
(4) Formation of the coat recruits a v-snare protein to the vesicle membrane.
(5) The GTP bound to the GTP-binding protein serves as a “small explosive charge” that will cause the coat to dis-assemble soon after the transport vesiclehas been pinched off the RER.Hsp70s help in the uncoating process! The GTP is hydrolyzed by the GTP-ase activity of the GTP-binding protein, which causes a conformational change in the protein. This change in shape of the GTP-binding protein (now with only GDP bound) causes a change in shape of the coatomer protein, which causes the coat to dis- assemble.
(6) With the coat removed, the v-snare protein is now exposed. (The term v- snare stands for vesicle-snare.)
(7) The v-snare is “snagged” by a t-snare protein in the membrane of the cis- cisternal membrane. (the term t-snare stands for target-snare.)
(8) The vesicle fuses with cis-cisternal membrane.
(9) Before the GTP-binding proteins are incorporated into the coat of a new transport vesicle, GTP will displace the GDP from the binding site. This re-charges this “explosive” device.
RECEPTOR FOR THE GTP-BINDING PROTEIN CARGO-BINDING PROTEINS
CARGO PROTEINS MEMBRANE OF CIS- CISTERNA V-SNARE T-SNARE
LUMEN OF CIS-CISTERNA
CAPTURE OF TRANSPORT VESICLES BY THE CIS- CISTERNA: FIG. 3 THE ROLE OF THE V-SNARE AND THE T-SNARE 5 So we have got the cargo proteins to the cis-cisterna of a Golgi body. The cargo proteins in the diagram are water-soluble proteins but a similar process exists for the transport of integral membrane proteins. We will not discuss how the fusion of the transport vesicle with the membrane of the cis-cisterna, but you would be correct if you guessed that more proteins are involved.
Now, according to the standard model (as we said above) the cargo protein undergoes modifications in the cis-cisterna and then is packaged up into transport vesicles and shipped off to the next cisterna. But there are some serious problems with this model. Two of these problems are:
(1) Immunofluorescence labelling gives no evidence for t-snares that can capture COPII v-snares in the Golgi membranes, except for the membrane of the cis-cisterna. Without these t-snares, transport vesicles with a COPII vesicle v-snare will not be able to dock to the cisternal membranes.
(2) Some cargo that goes through the Golgi body is far too big to be put into small transport vesicles. In mammals such cargo includes the procollagen molecules secreted by fibroblasts and osteocytes, and even more convincingly the complex scales formed by various protozoans.
EXOCYTOSIS
SECRETORY VESICLE
SCALES
TRANS-CISTERNA (aka TGN) ?????
CIS-CISTERNA (aka CGN)
PLASMA MEMBRANE ...... TRANSPORT VESICLES .. . RER
FIG. 4
SCALE FORMATION IN A PROTOZOAN
So there is clearly a problem moving the developing scale from one Golgi cisterna to the next!
Leblond had put forward another model called the cisternal maturation model, but Palades’ shuttling vesicle model took hold instead. But now Leblond’s model is being heavily 6 considered as being more likely, or at least as one model that must be correct. In the cisternal maturation model the cargo proteins (or scales) do not leave the cis-cisterna. Instead the cis-cisterna developes sequentailly into the later stage cisternae. We have said that each cisterna, as seen in the electron microscope, has a different set of modification enzymes. The cisternal maturation model holds that these enzymes move retrograde (backwards) up the Golgi stack. These vesicles are coated transport vesicles bit they have a somewhat different coatomer protein, and they are called COPI transport vesicles. Wouldn’t it make more sense to call these backward moving vesicles COPII vesicles, and the forward (anterograde) moving vesicles the COPI vesicles. Well it would. The problem is that the people who named them were wrong in terms of which direction the respective vesicles move.
CIS-CISTERNA
SECRETORY VESICLES
COPI I TRANSPORT VESICLES TRANS-GOLGI NETWORK
COPI TRANSPORT VESICLES
We does this model differ from the standard vesicular shuttle model we discussed at first?
(1) Transport vesicles still take cargo proteins to the cis-cisterna (or more accurately the cis-cisternal network). But that is the only anterograde (forward) movement of transport vesicles down the Golgi body.
(2) The transport vesicles that do move along the Golgi body are moving retrograde (backward) and they are COPI not COPII vesicles.
(3) These vesicles move from the cisterna just “upstream” from the trans-Golgi cisterna (more accurately the trans-Golgi network) to the cisterna just upstream from that and fuse with it. (4) These retrograde vesicles are not carrying cargo proteins, they are carrying the enzymes that are no longer needed by the cisterna they have just left. That cisterna now develops (matures) into the trans-Golgi cisterna.
(5) And so it goes up the Golgi body.
7
In non-animal cells the Golgi bodies can produce lots of carbohydrate, including cellulose
We have said that the Golgi bodies can add sugars to cargo proteins, resulting in glycoproteins. (This process of glycosylation actually begins in the RER.) But Golgi bodies of non-animal cells can produce lots of carbohydrate that is not attached to proteins. For example, the scales of protozoans can consist of 90% carbohydrate that is not bound to protein. So the concept that Golgi bodies are only involved in the modification of proteins is an animal, especially mammal, bias!