Deans Community High School Intermediate 2 Revision Notes www.evans2chemweb.co.uk
www.evans2chemweb.co.uk
User name : deans
Password : chemok
Unit 1 Building Blocks
The elements
In our world today, there are millions of substances in everyday use - liquids ranging from our drinking water to the fuel used in our cars, solids ranging from the sugar that we have on our breakfast cereal, and gases ranging from the air that we breathe to the gas we use to cook our food.
All of these substances are made from just over 100 different elements (103 to be precise). Of these elements, approximately 90 are naturally occurring and can be found in the earth's crust, the ocean and in our atmosphere. The remaining elements are not naturally occurring and are classed as man made.
Elements are the building blocks of substances - they cannot be broken down into anything simpler.
Chemical names and symbols Each chemical element is uniquely identified by it's chemical name and symbol.
The element Oxygen has the symbol - O The element Sodium has the symbol - Na The element Americium has the symbol - Am
Some elements have symbols that are derived from their common name (e.g. O for Oxygen), other elements have symbols that are derived from the word used for them in ancient times or foreign countries (e.g. Na is short for Natrium - the latin word for the element we know as sodium), other are named after famous scientists (e.g. Einsteinium) and countries or places of discovery (e.g. Americium).
Element symbols either have one or two letters only. The first is always an uppercase (capital) letter and the second is always a lowercase letter.
Classifying elements
Elements can be classified in several different ways e.g. as metal / non-metal, solid / liquid / gas, naturally occurring / man-made, radioactive / non-radioactive.
Elements are arranged into the Periodic Table.
The periodic table below shows the metals and the non-metals in the periodic table.
Metals are found to the left of the diagonal zig-zag line and non-metals are found to the right. A simple test to determine whether an element is a metal or non-metal is electrical conductivity.
All metals conduct electricity at room temperature. Non-metals do not conduct electricity at room temperature. An exception to this rule is that the element carbon in the form of graphite conducts electricity at room temperature.
The Periodic table below shows the physical state of the elements at 20°C. To help you decide whether an element will be a solid, liquid or gas at room temperature (20°C) look at the Periodic table (in the Data book) which shows melting and boiling points.
If the melting point and boiling point are both < 20°C then the element will be a GAS If the melting point < 20°C and boiling point > 20°C then the element will be a LIQUID If the melting point and boiling point are both > 20°C then the element will be a SOLID
N.B. Mercury (a metal) and Bromine (non-metal) are the only examples of liquids at room temperature.
Elements/substances that are solids are often given the suffix (s) - e.g. Ca (s) or Calcium (s) Elements/substances that are liquids are often given the suffix (l) - e.g. Hg (l) or Mercury (l) Elements/substances that are gases are often given the suffix (g) - e.g. Ar (g) or Argon (g)
This Periodic table tells you the symbols of some of the naturally occurring and man-made elements.
Some naturally occuring elements are radioactive (e.g. radium). Many of the man-made elements are radioactive and those above element number 92 are made inside nuclear reactors.
A horizontal row of elements in the Periodic Table is called a PERIOD. The vertical columns in the Periodic table are called GROUPS, and are shown below. The groups run from Group 1 to Group 7, followed by Group 0.
Elements in the same group of the Periodic table have similar chemical properties. The Alkali metals (Group 1) all react quickly with water to make an alkali and hydrogen - they are stored under oil. The Halogens (Group 7) are all reactive non-metals which react with metals to make salts. The Noble gases (Group 0) are all very unreactive elements. The Transition metals (between Groups 2 and 3) are reactive metals. These metals are often used as catalysts.
Chemical reactions.
Elements can undergo Chemical reactions. When elements react together a new substance is always formed. The substances formed when elements join together in a chemical reaction are called Compounds. Compounds can also undergo chemical reactions, and again a new substance is always formed.
We can usually tell that a chemical reaction has taken place if :
1. There is a change in appearance.
This can often be seen if the substance changes colour, or if a gas is given off during the reaction, or a precipitate is formed.
2. There is a change in energy
The simplest way to detect an energy change in a reaction is to measure the change in temperature. A change in energy can also be shown by light being produced or a noise, e.g. an explosion.
Reactions that give energy out are called EXOTHERMIC reactions. Reactions that take energy in are called ENDOTHERMIC reactions.
Naming compounds.
When elements react together, they form a new compound. The name of this new compound is derived from the elements that form it. 1. The elements in the compound are usually placed according to their position in the periodic table. Elements that are further to the left of the periodic table are written before elements that are further to the right of the periodic table. 2. If there are only two elements in the compound, the second part of name of the compound will end in -IDE. 3. If the compound contains 'lots' of oxygen as well as two other elements, then the ending of the second part of the compound name will be -ATE. 4. If the compound contains 'a smaller' amount of oxygen (less than for in the corresponding -ATE compound) as well as two other elements, then the ending of the second part of the compound name will be -ITE.
Word equations.
The reactions between elements (or compounds) can be shown by use of a word equation. This is used to show the REACTANTS and the PRODUCTS.
REACTANTS PRODUCTS
1. Reactants are the substances that have undergo a chemical reaction together and are shown on the left of the word equation. 2. Products are the substances that are formed as a result of a chemical reaction and are shown on the right of the word equation. 3. When writing a word equation, we use the symbol (meaning 'reacts to give') and not the = sign. The reason is that if we used an equal sign, we are saying that the products are the same as the reactants. This is not the case, as all chemical reactions produce a new substance.
For example, the reaction between sodium metal and chlorine gas would be :
sodium + chloride sodium chloride
Mixtures
When elements or compounds come together but do not react, a MIXTURE is formed.
The air that we breathe is a mixture of the elements oxygen (21%), nitrogen (78%), and various other gases (1%) that have not reacted together.
The test for oxygen is that it relights a glowing splint. However, there is not a high enough percentage of oxygen in the air to test this.
Substances that exist in a mixture can be separated by physical methods (e.g. filtration, distillation, chromatography, etc.). Chromatography - can be used to separate small quantities of similar substances e.g. dyes in coloured ink.
Solutions.
Solutions are formed when a substance dissolves in another substance.
The substance that is dissolved is called the SOLUTE and the substance that it dissolves in is called the SOLVENT.
If the solute dissolves in the solvent, it is said to be SOLUBLE. If the solute does not dissolve in the solvent, it is said to be INSOLUBLE.
When a solution is made with very little solute present (or a large amount of solvent), it is said to be a DILUTE SOLUTION. When a solution is made with a lot of solute present (or a small amount of solvent), it is said to be a CONCENTRATED SOLUTION. When a solution is made with the maximum amount of solute that will dissolve, it is said to be a SATURATED SOLUTION.
Substances that are in solution are often given the suffix (aq) - e.g. NaCl (aq) or Sodium chloride (aq)
The term aq refers to the solute being dissolved in the solvent water - i.e. it is an AQUEOUS SOLUTION.
To dilute a concentrated or saturated solution, more solvent should be added. To make a dilute solution a concentrated one, solvent should be removed (usually done by boiling the solution), or more solute should be added. To make a solution a saturated one, more solute should be added until no more can dissolve, or solvent should be removed until the solute begins to come out of the solution.
NEW WORDS AND THEIR MEANINGS
SUBSTANCE - a general name for a chemical.
ELEMENT - the simplest type of chemical substance.
NATURALLY OCCURRING ELEMENTS - elements that can be found in nature (in the earth's crust, oceans or air).
MAN-MADE ELEMENTS - elements that do not occur in nature and are synthesised in the laboratory.
PERIODIC TABLE - a table of all the known elements sorted into vertical groups and horizontal periods.
ELECTRICAL CONDUCTIVITY - the ability of a substance to allow electricity to flow through it. All metals are conductors of electricity, and non-metals do not conduct electricity (except carbon in the form graphite).
ALKALI METALS - the elements in group 1 of the periodic table. They are reactive metals. HALOGENS - the elements in group 7 of the periodic table. They are reactive non-metals.
NOBLE GASES - the elements in group 0 of the periodic table. They are very unreactive gases.
TRANSITION METALS - the elements found between group 2 and 3 of the periodic table. They are reactive metals often used as catalysts.
COMPOUND - formed when two or more elements react together.
EXOTHERMIC - a chemical reaction that gives out energy (usually heat) to its surroundings. This usually means the reaction heats up.
ENDOTHERMIC - a chemical reaction that takes in energy (usually heat) from its surroundings. This usually means the reaction cools down.
MIXTURE - formed when substances a placed together but do not react with one another.
SOLUTION - formed when a SOLUTE dissolves in a SOLVENT.
Using reaction rates to follow the progress of a reaction.
The progress of a chemical reaction can be followed by examining the reaction rate. There are several methods that can be used to follow a reaction rate.
Change in colour of a reaction Change in concentration of reactants or products Change in volume of any gases produced Change in mass of reaction mixture if gases are given off or taken in. etc.
As these changes happen over a period of time, the reaction rate is expressed as a change in some measurable quantity over a period of time.
For example, the reaction rate could be measured by examining the change in concentration of a product over a certain time - in other words, the difference between the final concentration of a product and the initial concentration of the product taken over a measured period of time.
Mathematically, this would be written as :
Change in concentration Reaction rate = Time taken for change
The units of the rate of a reaction depend on the method used to calculate the rate. The simplest way to determine the units is to put them into the calculation used to find the reaction rate.
For example, if we measured the change in concentration over time in order to obtain the rate of the reaction, the units would be as follows :
The unit of concentration is moles per litre, which is written as mol l-1. The unit of time is seconds, written as s
Putting these units into the above equation gives : mol l-1 Reaction rate = s = mol l-1 s-1
In the same way, a reaction rate measured by the change in volume of a gas produced (measured in cm3) over a period of time (measured in s), would have the units cm3s-1. A reaction rate measured by the change in mass of a reaction would have the units g s-1.
The progress of a chemical reaction can be illustrated by the use of a graph. These graphs show that reaction rates are usually highest at the beginning of the reaction (shown by a steep slope). This is because as a reaction proceeds, the reactants are used up and the reaction begins to slow down.
If we measure the change in concentration of a reaction by the concentration (or volume, or mass) of products, this value increases as the reaction proceeds. If we measure the change in concentration of a reaction by the concentration (or volume, or mass) of reactants, this value decreases as the reaction proceeds.
The average reaction rate is proportional to 1/time.
When a reaction is quick (small time), the reaction rate is high. When a reaction is slow (large time), the reaction rate is low.
Collision Theory
For substances to react together, they first have to collide with one another. This idea is called the Collision Theory.
This theory can be used to explain why certain factors can affect the rate of a reaction.
Factors affecting reaction rate - Concentration
The more particles there are in a given space, the more likely they are to collide with one another.
These animations illustrate that the more concentrated a reaction mixture, the more likely the reactants are to collide with one another and hence an increase in the rate of the reaction.
Not all collisions result in a chemical reaction taking place - the collisions must take place with sufficient energy to break any bonds within the reactants. For example nitrogen and oxygen particles collide in the air very frequently, but do not normally undergo a chemical reaction as there is insufficient energy in the collisions.
Factors affecting reaction rate - Surface area
If a large piece of material (1 particle) has a size of 2cm x 2cm x 2cm, the surface area of the reactant will be 24cm2. This means that any particles that are trying to react with this material have to collide somewhere on this surface of 24cm2.
If this large piece of material was broken up into eight pieces of 1cm x 1cm x 1cm, there would now be a surface area of 48cm2. This means that for this reaction, any particles that are trying to react with this material have a much larger surface area (or target area) to hit - they are more likely to collide and react with one another.
Also, as can be seen in the animation for the effect of concentration, one particle is less likely to collide with another particle than 4, or even 8 particles are.
Factors affecting reaction rate - Temperature
As reactants are heated up, they begin to move more rapidly. As can be seen in the animations below, the faster the particles move, the more frequently they collide, meaning they are more likely to react with one another.
Low temperature High temperature Also, as the particles are now moving faster, the collisions between them have more kinetic energy and as a result, they are more likely to be successful collisions that cause a chemical reaction.
Catalysts
Catalysts are used to speed up a chemical reaction. They are not used up in the reaction and can be recovered chemically unchanged at the end of the reaction.
Many catalysts are elements (or compounds of) found between groups 2 and 3 of the Periodic Table - The Transition metals (This was mentioned in Unit 1 : Substances).
Hydrogen peroxide decomposes (breaks up) slowly releasing oxygen gas. If manganese dioxide is added the reaction is much faster and oxygen gas is given off quickly. Manganese dioxide is a catalyst for this reaction. At the end of the reaction all of the manganese dioxide can be recovered (by filtration) and used again.
Catalysts help to increase the rate of a reaction by increasing the percentage of successful collisions between reactants without increasing the surface area, concentration or temperature.
How does a catalyst work ?
For a chemical reaction to occur, the bonds within the reactants have to be broken before new ones can been formed with other reactants. When reactants simply collide with one another, there is not always enough energy in the collision to break the bonds. When a catalyst is used, the reactants adsorb onto the surface of the catalyst. Because the reactants use some of the energy from their existing bonds to form a new bond with the catalyst, the original bonding in the reactants is slightly weakened. This reduces the energy needed during a collision to break these bonds and for a reaction to take place.
After the reaction has taken place, the new product breaks its bond with the catalyst and leaves the surface.
What types of catalyst can be used ?
A very common source of catalysts are the Transition metals. When the reactants are gases, a solid catalyst is usually used. A catalyst that is in a different physical state (solid, liquid or gas) than the reactants is called at Heterogeneous Catalyst - e.g. a platinum gauze being used as a catalyst in the formation of nitric acid from ammonia and oxygen in the Ostwald Process. A catalyst that is in the same physical state (solid, liquid or gas) as the reactants is called a Homogeneous Catalyst.
Uses of catalyst.
As we have seen, reactions are more likely to take place when high concentrations, large surface areas and high temperatures are used. These factors increase the likelihood of collisions of the reactants, and the more energy that these collision have, the more likely it will be that these collisions are successful and cause a chemical reaction to take place.
When chemical processes are performed on a large scale in industry, the costs can be extremely high if the reactions require a large amount of energy (usually in the form of heat). Also some reactants and products actually begin to decompose or react in different ways if the temperature is too high; so although the temperature gives the collisions enough energy to cause a chemical reaction, the product may decompose before it can be isolated.
Catalysts reduce the energy required for a reaction to proceed and as a result usually require much lower temperatures (cheaper), and more collisions are successful (efficient).
Catalysts are also used in car exhausts to convert harmful gases such as carbon monoxide to carbon dioxide and oxides of nitrogen to nitrogen.
Occasionally Catalyst Poisoning can take place if impurities are adsorbed onto the surface of a catalyst. When this happens, the impurities block the sites on the catalyst that the reactants adsorb onto. This reduces the efficiency of the catalyst, and can stop it working completely if the entire surface is coated with impurities.
Some poisoned catalysts can be Regenerated by removing the impurities from the surface, but other catalysts have to be Renewed if the impurities cannot be removed or if the surface has been damaged.
An example of catalyst regeneration is the removal of carbon (by burning it off in air) from the aluminium oxide catalyst used in the catalytic cracking of long chain hydrocarbons.
In a car exhaust, lead can poison a catalytic convertor and this is why only lead-free fuels should be used.
Enzymes
Another type of catalyst is a biological catalyst, or Enzyme.
Enzymes are very specific in the type of reaction that they will catalyse, and these reactions take place in living material - e.g. the human body, animals and plants. They are examples of homogeneous catalysts as they are found in a solution with the reactants.
Some examples of enzymes are :
Saliva contains an enzyme called amylase which helps the digestion of certain foods. During the baking and brewing process, yeast (which contains an enzyme called zymase) breaks down sugars into carbon dioxide (gas) and alcohol.
NEW WORDS AND THEIR MEANINGS REACTION RATE - The speed at which a reaction takes place.
COLLISION THEORY - Reactant particle need to collide to react with one another.
KINETIC ENERGY - The energy a moving particle has. Fast, heavy particles have higher kinetic energies than slow, light particles.
CATALYST - A substance used to speed up a chemical reaction. Catalysts can be recovered chemically unchanged at the end of a reaction.
HETEROGENEOUS CATALYST - A catalyst that is in a different physical state than the reactants.
HOMOGENEOUS CATALYST - A catalyst that is in the same physical state as the reactants.
CATALYST POISONING - The surface of a catalyst can be poisoned, or smothered by an impurity, rendering it useless.
CATALYST REGENERATION - Poisoned catalyst can be cleaned to remove impurities, allowing it to be used again.
CATALYST RENEWAL - Catalyst that cannot be cleaned are discarded and replace with new ones.
ENZYME - A biological catalyst.
Atoms
In Unit 1 - Substances, we learned that an element is the simplest type of substance. Each element is made up of atoms. Atoms are tiny particles that are specific to each element.
e.g. there are approximately 150500000000000000000000 atoms in 1g of helium gas!
Sub-atomic particles
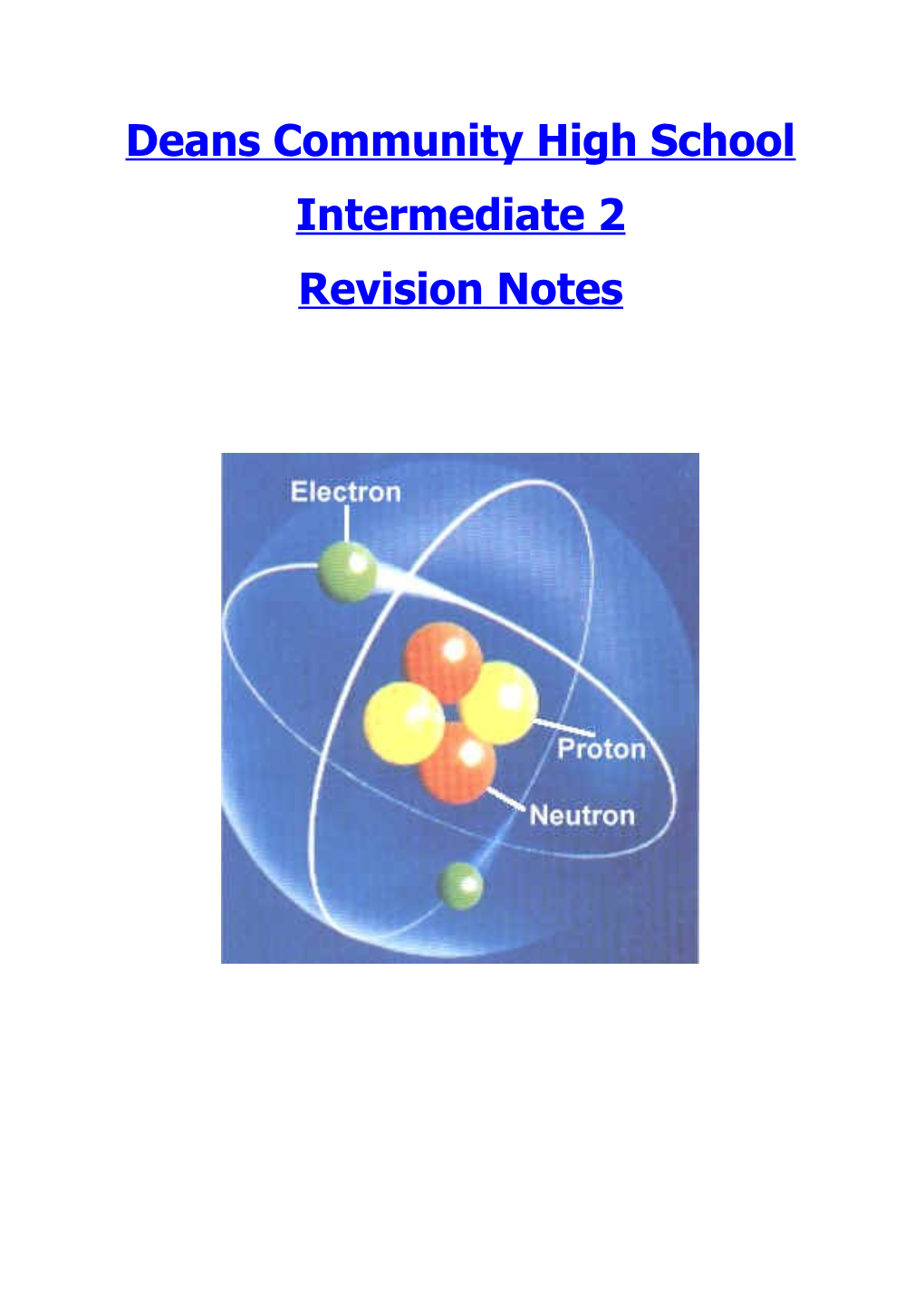
Although atoms were once thought to be solid, indivisible particles, they are now known to have a very small positively charged centre, called the nucleus with negatively charged electrons moving around in the space around the nucleus as shown.
By examining α (alpha) scattering plots, scientists discovered that atoms consist of a very small positively charged nucleus surrounded by mostly empty space. They discovered the centre of the atom, which is called the nucleus, was positively charged, and consisted of two particles - The proton and the neutron.
Around the nucleus was a negatively charged area that contained paricles called electrons.
The mass of sub atomic particles are measured in atomic mass units (amu).
The table below shows as summary of the sub-atomic particles :
Particle Charge Mass Position in the atom Proton +1 1 amu Found in the nucleus Neuton 0 1 amu Found in the nucleus Electron -1 0 amu (approx) Found around the nucleus (in the electron cloud)
In every atom, the number of protons (positively charged) is exactly the same as the number of electrons (negatively charged) otherwise the atom would have a charge associated with it.
Electron arrangement
The electrons around the nucleus are arranged into different energy levels or shells.
Each shell can only hold a maximum number of electrons.
The first energy level can hold up to 2 electrons The second energy level can hold up to 8 electrons The third energy level can hold up to 8 electrons
Electrons fill up these energy levels sequentially - i.e. starting at the first energy level and working up. The electron arrangement of an atom is determined by placing the electrons into the correct energy levels, and is written as :
a,b,c.....
Where
a is the number of electrons in the first energy level b is the number of electrons in the second energy level c is the number of electrons in the third energy level etc..
When an energy level is empty it is not written, e.g. electron arrangement of neon is 2,8 (not 2,8,0).
Elements that have the same number of outer shell electrons (highest energy level that contains electrons) have similar chemical properties and are found in the same group of the periodic table.
For example, calcium (2,8,8,2) and magnesium (2,8,2) have similar chemical properties to each other because they both have 2 electrons in their outer energy levels.
The diagrams below show a graphical representation of the energy levels or shells of several atoms, with the electron arrangement given below each.
Hydrogen Fluorine Oxygen Nitrogen Carbon
1 2,7 2,6 2,5 2,4
Electron arrangements are given in the data book, but can be worked out as follows :
If an atom has 9 electrons, the electron arrangement would be :
2 electrons in the first shell, which can hold a maximum of 2 (leaving 9-2 = 7)
7 electrons in the second shell, which can hold a maximum of 8
The electron arrangement of this atom is 2,7
If an atom has 11 electrons, the electron arrangement would be :
2 electrons in the first shell, which can hold a maximum of 2 (leaving 11-2 = 9)
8 electrons in the second shell, which can hold a maximum of 8 (leaving 9-8 = 1)
1 electron in the third shell, which can hold a maximum of 8
The electron arrangement of this atom is 2,8,1
Atomic number
The periodic table is arranged by the atomic number of each element. The atomic number is the number of protons in an atom of this element The atomic number defines the element Since an atom is neutral, it is also the same as the number of electrons in the atom These are whole numbers
Mass number
The mass number is the defined as the sum of the number of protons and neutrons in an atom
A carbon atom has a mass number of 12, and an atomic number of 6 (=6 protons and 6 electrons). It must have 6 neutrons as 6 protons + 6 neutrons = 12 (mass number)
Atomic number = protons = electrons Mass number = protons + neutrons Neutrons = Mass number - Atomic number Neutrons = Mass number - protons
Nuclide Notation
This is a way of showing mass number, atomic number and symbol for an element. An example is shown on the right.
From this information, the following can be determined for this atom of chlorine:
Atomic number = 17 Mass number = 35 Number of protons = 17 Number of electrons = 17 Number of neutrons = 35-17 = 18
Isotopes and relative atomic mass
There are two type of chlorine atoms as shown below.
These are chemically identical because they have the same number of protons and the same electron arrangements (number of electrons), but they have different mass numbers. This is because the chlorine-37 atom has two more neutrons than the chlorine-35 atom. This makes the chlorine-37 atom heavier. These two forms are called isotopes - atoms with the same atomic number but with a different mass number. Most atoms have isotopes.
Atoms are compared in mass to a carbon atom with a mass number of 12, and which is given a mass of 12 atomic mass units (a.m.u.).
A hydrogen atom has only 1/12 of the mass of this carbon atom and so has an atomic mass of 1 a.m.u.
Some chlorine atoms have a relative atomic mass of 35 a.m.u. while others are heavier with a mass of 37 a.m.u.
When chlorine atoms are compared in mass to a carbon atom with a mass number of 12, an relative atomic mass of 35.5 is obtained for chlorine.
This value is between the mass of the two isotopes but it is closer to chlorine-35 than to chlorine-37 as there are more atoms weighing 35 a.m.u. than 37 a.m.u.
As most atoms have isotopes, average atomic masses of elements are rarely whole number values.
NEW WORDS AND THEIR MEANINGS
ATOM - Small particles that make up an element.
NUCLEUS - The small centre of every atom. The nucleus contains the positive protons and the neutral neutrons. Virtually all of the mass of the atom is found here.
PROTON - Positively charged particles found in the nucleus. They have a mass of 1 amu.
NEUTRON- Neutral particles found in the nucleus alongside the protons. They have virtually the same mass as a proton (1 amu)
ELECTRON - Negatively charged particles found around the nucleus in an atom. They have virtually no mass (0 amu).
ELECTRON ARRANGEMENT - The ordering of an atoms electrons into energy levels or shells.
ATOMIC NUMBER - The number of protons in an atom (also equal to the number of electrons in the atom). Atomic number determines where the element is found in the periodic table.
MASS NUMBER - The sum of the protons added to the neutrons.
ISOTOPES - Atoms with the same atomic number but with a different mass number (Atoms of the same element with a different number of neutrons).
RELATIVE ATOMIC MASS - The average mass of atoms of the element in question, taking into consideration the mass and relative abundance of isotopes of that element.
Why do atoms bond ? As you have learned in the 'Structure of the atom' topic, electrons are placed into energy levels, and the electron arrangement can be given for each atom. The most stable compounds in the periodic table, are the Noble gases (you learned this in the 'substances' topic). The electron arrangement of these gases all have the maximum number of electrons in their outer shell.
Helium Neon Argon
Electron arrangement : 2 2,8 2,8,8
Scientists believe that when the outer electron shell is full, this is the most stable arrangement of electrons and is called a stable electron arrangement . When atoms bond to each other, they attempt to achieve the same electron arrangement of the nearest noble gas. They do this in several different ways and only the outer shell electrons are used in forming bonds.
The covalent bond
As with all bond formations, the atoms in question must first collide with one another (as mentioned earlier in the 'Reaction Rates' topic).
When some atoms collide with each other, the electrons in the outer shell can be shared between the atoms. Although the electrons of the two atoms are both negatively charged and repel each other, when a collision takes place with sufficient energy to form a compound, the outer energy levels overlap and the atoms share the electrons. The overlap area has an increase in negative charge, which is strongly attracted by the positive nuclei of both atoms. This effectively draws the two atoms closely together and a strong force of attraction between the nuclei and the shared electrons forms a strong covalent bond.
Covalent bonds only occur between non-metal atoms and the bond is a strong force of attraction.
When covalent bonds are formed between a small number of non-metal atoms, the resulting compound is called a molecule.
Some examples showing the sharing of outer shell electrons to obtain a stable electron arrangement are :
Electron clouds
In the 'Structure of the atom' topic, you learned that electrons are found in the space around the nucleus of the atom. You also learned in the 'Structure of the atom' topic, that an atom's electrons are ordered into energy levels or shells.
Within these shells, the electrons are found in electron clouds or orbitals.
Electron clouds have different shapes depending on the energy level the electrons are in. The shape of the electron cloud defines the area in which its electrons can be found Each electron cloud can hold a maximum of 2 electrons Electrons fill these clouds / orbitals singly if possible, but they double up after each empty cloud on the energy level contains one electron. Electrons normally only move onto the next energy level once the current energy level is full.
Some electron clouds are shown below :
2nd energy level 3rd energy level 1st energy level (with 4 or more electrons) (with 4 or more electrons)
1 electron cloud 4 electron clouds 4 electron clouds (1x2)=2 electrons (4x2)= 8 electrons (4x2)= 8 electrons
For example, a hydrogen atom, which has 1 electron, has the electron arrangement 1.
This electron is in the first energy level which is in the shape of a sphere.
(In the diagrams that follow, electrons are indicated by the small dots inside the orbitals) For example, an oxygen atom, which has 8 electrons, will have the electron arrangement, 2,6
This means that two electrons are in the inner shell (sphere shaped) and the other six electrons are in the outer shell (in the shape of a tetrahedron). This is shown below.
2D representation of 3D representation of 1st and 2nd energy levels 2nd energy level only
Polar covalent bonding
When covalent bonds are formed, the electrons that are shared between the two atoms are not always equally shared. This is because some atoms attract electrons more strongly than others. This attraction is called electronegativity.
The only type of covalent bond that has an equal share of electrons between the two atoms is when the two atoms are of the same element. e.g. H2, O2, Cl2. These bonds are called pure covalent bonds.
All other covalent bonds are classed as polar covalent bonds.
For example, the molecule water has two elements : oxygen and hydrogen. The oxygen atom attracts the electrons more strongly than the hydrogen atoms and as a result, pulls the shared electrons closer to itself. This makes the oxygen atom slightly more negatively charged (δ-) than the hydrogen atoms (δ+). This creates a permanent dipole (meaning two poles or charges on the molecule).
When molecules contain polar covalent bonds, the molecules have a slightly positive end which can attract the negatively charged ends of other molecules and vice-versa. The interactions of permanent dipoles between water molecules Molecules that have polar covalent bonding are classed as polar molecules.
This type of attraction is weak compared to the covalent bond.
Discrete covalent substances
Discrete covalent substances are made up of molecules.
When a molecule contains only two atoms (e.g. H2, Cl2, HCl), it is called a diatomic molecule. All of the Halogens (group 7) exist as diatomic molecules, as do Hydrogen, Nitrogen and Oxygen.
When a molecule contains only three atoms (e.g. CO2, H2O, O3), it is called a triatomic molecule.
All discrete covalent substances (with polar and non- polar bonding) exhibit a type of attraction between the molecules. This attraction is called van der Waals forces.
This attraction arises when random electron movement within the orbitals causes one side of a molecule to have a slightly negatively charged end and a slightly positively charged end (a temporary dipole). These charges cause molecules to attract one another in the same way that a polar molecule does. This force of attraction is much The interactions of temporary smaller than that in a covalent bond. dipoles between molecules
Because these charges (or dipoles) are randomly distributed and are not permanent, this type of van der Waals force is usually smaller the attraction between polar molecules.
Multiple covalent bonds
Sometimes an atoms can form more than one bond with each other in an attempt to achieve a stable electron arrangement. e.g. Oxygen has an electron arrangement of 2,6 and requires a share of a further 2 electrons to reach the electron arrangement of Neon (2,8).
Oxygen exists as a diatomic element, i.e. it forms covalent bonds with itself. Since each oxygen atom is short of two electrons, it can form two covalent bonds with the other atom. This is called a double bond and involves the sharing of 4 electrons between two atoms.
A double bond between oxygen atoms forming a molecule of oxygen e.g. Nitrogen also exists as a diatomic element. This involves two nitrogen atoms sharing 6 electrons with each other (three from each atom) forming a triple bond.
A triple bond between nitrogen atoms forming a molecule of nitrogen Elements that require four electrons to achieve a stable electron arrangement do not form 4 bonds with each other as there is too much strain involved in the bond. Instead they can form a combination of single, double and triple bonds to gain a stable arrangement.
The shapes of molecules
The shapes of molecules are based on the shapes of the orbitals that are used in bonding.
When a carbon atom (electron arrangement - 2,4) bonds to four hydrogen atoms to form a compound called methane, or carbon hydride, the carbon shares an electron with each of the four hydrogen atoms to achieve the same electron arrangement as Neon (2,8), and the four hydrogen atoms also achieve a stable arrangement of Helium (2) as shown on the left.
Since carbon's bonding electrons are in the second energy level (2,4), they are arranged in the shape of a tetrahedron as shown on the right.
When this atom of carbon bonds to four hydrogen atoms the molecule has the shape of a tetrahedron and looks like this:
3D representation of the shape 3D representation of the shape of a molecule of methane of a molecule of methane and shapes of orbitals used
When a nitrogen atom bonds (covalent bonds) with three hydrogen atoms (to form a molecule of ammonia), the resulting molecule shape is called a pyramidal structure and is shown below :
Notice that there is a pairs of electrons that are not involved in the formation of the three single covalent bonds. These electrons are called lone pairs, and they take up one of the tetrahedral positions and affect the shape of the molecule.
When an oxygen atom bonds (covalent bonds) with two hydrogen atoms (to form a molecule of water), the resulting molecule shape is called a bent structure and is shown below (the oxygen atom has two lone pairs of electrons that are not involved in bonding) :
Chemical formulae and structural formulae
The chemical formula tells us the elements that are in a compound and it also tells us the number of each type of atom that is present.
For example, the compound hydrogen oxide (or water) contains hydrogen and oxygen only (you learned this in the 'Substances' topic).
You also know from the previous examples that oxygen needs to share an electron with two hydrogen atoms to achieve a stable electron arrangement. This means that there are two hydrogen atoms for every one oxygen atom.
This gives the chemical formula of hydrogen oxide / water as H2O.
Notice that the number '1' (showing one atom of oxygen) is not written in the chemical formula.
The full structural formula of a compound also tells us the elements present and the number of atoms of each, but in addition tells us the way that these atoms are arranged in the compound.
The chemical formulae and full structural formulae of some other compounds are shown below :
Chemical name Atoms present Chemical Formula Full Structural Formula
Hydrogen oxide (water) H & O H2O
Nitrogen hydride (ammonia) N & H NH3
Carbon hydride (methane) C & H CH4
Ethanol (alcohol) C, H & O C2H5OH
Covalent networks
As well as discrete covalent compounds and small molecules, bonds can be formed between many atoms forming a giant compound containing many hundreds, thousands, or millions of atoms.
These compounds are called covalent networks and consist of a giant lattice of covalently bonded atoms.
For example, the element carbon can exist in the form of diamond (as you learned in the 'Substances' topic), which is a covalent network structure.
The full structural formula of diamond is nearly impossible to write as it contains a huge number of carbon atoms.
In the same way, the compounds silicon carbide and silicon oxide form giant lattices / covalent networks. Part of the structures of these are shown below :
Since these substances contain a huge number of atoms, it is not practical to write the chemical formula for them. Instead we write the empirical formula .
The empirical formula shows us the simplest ratio of elements in the substance.
For example : The empirical formula of silicon carbide (1 silicon atom for every 1 carbon atom) is SiC
The empirical formula of silicon oxide (1 silicon atom for every 2 oxygen atoms) is SiO2
For a covalent network, the chemical formula is written as the empirical formula.
Ionic bonding
An ionic bond (sometimes called an electrovalent bond) usually occurs between a metal and a non- metal and involves ions, which are charged atoms (or groups of atoms).
In ionic bonding, electrons are donated from one atom to another allowing both atoms to achieve a stable electron arrangement.
If we consider the group 1 metals, they have only have one electron in their outer shell. In order to achieve a stable arrangement, these metals 'lose' their single outer shell electron, leaving the next lower energy level full. For example, the element sodium (electron arrangement 2,8,1) can lose its outer shell electron to achieve the electron arrangement 2,8. This leaves a 1+ (one positive) charge on the atom and this is called a positive ion.
Some metals (group 2) have 2 electrons in their outer shell and lose these to gain a stable electron arrangement. When this occurs they have a charge of 2+.
Similary aluminium can lose 3 outer shell electrons to achieve a stable electron arrangement leaving a 3+ charge on the atom.
Some examples of positive ions are shown in the table below.
Metal Electron arrangement No. electrons to lose Ion Lithium 2,1 1 Li+ Sodium 2,8,1 1 Na+ Magnesium 2,8,2 2 Mg2+ Aluminium 2,8,3 3 Al3+
In the same way, non-metal atoms can also gain electrons from the metal in order to reach a stable electron arrangement. For example, chlorine which has an electron arrangement of 2,8,7 requires one electron to fill its outer shell, making it 2,8,8. This in turn gives the chlorine a negative charge as it has one more electron than protons in the nucleus. This is called a negative ion.
Some examples of negative ions are shown in the table below.
Non-metal Electron arrangement No. electrons to gain Ion Fluorine 2,7 1 F- Chlorine 2,8,7 1 Cl- Oxygen 2,6 2 O2- Nitrogen 2,5 3 N3-
When a metal gives its outer shell electron(s) to a non-metal, the positive ion and negative ions that are formed attract one another and form an ionic bond.
For example, sodium and chlorine atoms would form an ionic bond making the compound sodium chloride as shown below :
There are also ions that exist that contain more than one element. These are called group ions and 2- + some examples are the sulphate ion (SO4 ) and the ammonium ion (NH4 ). The formulae for these group ions are found in the data booklet. Ionic compounds often form lattices which are called ionic structures. These structures are held together due to attraction between neighbouring, oppositely-charged ions.
As with covalent network structures, the empirical formula is given rather than the chemical formula. A small portion of the Sodium chloride lattice Ionic bonds are extremely strong. The chemical formula is NaCl
Metallic bonding
Metals, as mentioned in ionic bonding, can lose their outer shell electrons to gain a stable electron arrangement. Metal atoms are arranged in a lattice and can also delocalise their outer shell electrons, allowing them to move freely between the atoms in the lattice.
This effectively brings the metals closer to obtaining a stable electron arrangement and since the metal atoms become positively charged (ions) they attract the free moving electrons in the lattice. This attraction forms a metallic bond which is very strong.
Properties of substances due to type of bonding
Electrical conductivity
A substance will conduct electricity when charged particles can move through the material. When this happens, the material is said to be an electrical conductor, and if the material does not allow the movement of charged particles through it, it is called and insulator.
Charged particles can be electrons or ions. When a material conducts electricity by allowing electrons to move through it, the material is not altered chemically. However, when a substance conducts electricity by allowing ions to move through it, these substances are usually broken up in the process (this is called electrolysis and is mentioned further down the page).
Covalent compounds do not conduct electricity as they do not contain any charged particles.
Ionic compounds contain ions and will conduct electricity when these particles can move freely through the material. This only occurs when the ionic compound is in solution or when it is molten (has been melted into a liquid). An ionic compound in solution or as a melt is called and electrolye. Solid ionic compounds do not conduct electricity.
All metallic elements conduct electricity as they have electrons moving freely through their structures.
Carbon in the form of graphite also has delocalised electrons in its structure and as a result will conduct electricity.
Melting points and boiling points
To melt a substance, the bonding between units (molecules and ionic structures) has to be substantially weakened (or partly broken). To boil a substance, these bonds have to be broken.
Note that the covalent bonds between atoms are not broken during melting or boiling, only the attraction between molecules and units - these forces of attraction are called van der Waals forces (present in all substances), those between polar molecules (covalent substances) and those between neighbouring ions (ionic compounds).
Discrete covalent substances have low melting and boiling points. Almost all substances (except mercury) that exists as gases or liquids at room temperature, are discrete covalent substances. Polar covalent substances have higher melting and boiling points than pure covalent substances due to the increased attraction between molecules. Covalent networks have high melting and boiling points and are all solids at room temperature. This is because the structure is held together by strong covalent bonds. Ionic compounds have very high melting and boiling points and are all solids at room temperature. This is because ionic bonds need to be weakened/broken to melt or boil them. Metallic elements have high melting and boiling points. This is because metallic bonds need to be weakened/broken to melt or boil them.
Colours of ionic compounds
The colours of ionic substances depends on the colour of the ions present.
The colours of some ions are listed below :
Ion Formula Colour Sodium Na+ colourless Potassium K+ colourless Copper Cu2+ blue Chloride Cl- colourless 2- Sulphate SO4 colourless 2- Chromate CrO4 yellow
For example, sodium chloride is likely to be colourless, copper sulphate is likely to be blue, potassium chromate is likely to be yellow and copper chromate is likely to be green.
Solubility
Most ionic substance dissolve in water and this involves the lattice being broken up completely.
Some covalent substances dissolve in water, but most dissolve in other solvents.
Usually the rule 'like dissolves like' applies - This means that :
non-polar solutes will dissolve in non-polar solvents polar solutes will dissolve in polar solvents ionic solutes will dissolve in polar solvents There is also an overlap, meaning that some non-polar solutes will dissolve in polar solvents (and vice-versa) and ionic solutes will dissolve in polar solvents (and vice-versa).
Electrolysis
Electrolysis is the use of electricity to break up an ionic compound either in solution or as a melt (an electrolyte).
Covalent compounds or metallic elements cannot undergo electrolysis.
Normally, a d.c. supply is used during electrolysis. One electrode is connected to the positive end of the supply and is called the anode. The other electrode is connected to the negative end of the supply and is called the cathode.
During electrolysis, the positive ions are attracted to the negative electrode and the negative ions are attracted to the positive electrode.
At the positive electrode, negatively charged non-metal ions lose electrons.
At the negative electrode, positively charged ions gain electrons.
For example, when a solution of copper chloride undergoes electrolysis, the copper ions are attracted to the negative electrode and the chloride ions are attracted to the positive electrode.
The copper ions gain two electrons and form copper metal, and the chloride ions lose two electrons to form chlorine gas.
Cu2+(aq) + 2e- Cu(s) - - 2Cl (aq) Cl2(g) + 2e
NEW WORDS AND THEIR MEANINGS
STABLE ELECTRON ARRANGEMENT - An atom is most stable when it has the electron arrangement of the nearest noble gas in the periodic table.
COVALENT BOND - Formed when atoms share electrons to achieve a stable electron arrangement.
ELECTRON CLOUD / ORBITAL - The space that an electron occupies in an atom. Each energy level has different electron clouds associated with it.
PURE COVALENT BOND - A covalent bond formed between two atoms of the same element. This means the atoms have an equal share of the electrons in the bond.
POLAR COVALENT BOND - A covalent bond formed between two atoms in which the atoms have an unequal share of the bonding electrons. The atom which has the greatest share has as δ- charge and the atom with the least share has a δ+ charge associated with it.
ELECTRONEGATIVITY - The strength with which an atom attracts electrons.
DISCRETE COVALENT SUBSTANCES - These are made up of small molecules. VAN DER WAALS FORCES - The forces of attraction between molecules (and atoms) caused by temporary positive and negative charges being formed due to random electron movement.
DIPOLE - Formed when a molecule (or atom) has a positively charged end and a negatively charged end. These can be permanent (as in polar molecules) or temporary (as in van der Waals forces caused by random electron movement).
LONE PAIR - Two electrons in an orbital that are not used in bonding.
CHEMICAL FORMULA - Tells us the atoms present and the quantity of each in a compound.
FULL STRUCTURAL FORMULA - Tells us the atoms present, the quantity of each and their arrangement in a compound.
COVALENT NETWORK - A giant lattice of covalently bonded atoms.
EMPIRICAL FORMULA - A type of chemical formula that gives the simplest ratio (not the actual numbers) of atoms in a compound.
IONIC BOND - A bond that involves the transfer of electrons from one particle to another to form positively and negatively charged particles. These particles attract one another and form an ionic bond.
IONS - A charged atom (or group of atoms) : A positive ion is an atom (or group of atoms) that has lost electrons and a negative ion is an atom (or group of atoms) that has gained electrons.
METALLIC BOND - A bond formed between metal atoms, where the outer shell electrons are delocalised and allowed to move freely throughout the lattice of metal atoms.
ELECTROLYSIS - The breaking down of a compound using electricity.
Valency
In the previous topic, 'Bonding, structure and properties', you came across various types of bonding that allowed atoms of different elements to react together and achieve a stable electron arrangement, namely Covalent bonding and Ionic bonding.
In both of these types of bonding, the atoms needed to lose or gain a certain number of electrons in order completely fill their outer shell. This number is called the Valency and is useful in determining the chemical formula of the compound that this atom will form when it reacts with another atom.
The valency of an atom is also called its Combining power. e.g. The non-metal oxygen has an electron arrangement of 2,6
This means that oxygen needs to gain two electrons to achieve a full outer shell.
The valency of oxygen atom is therefore 2 e.g. The metal sodium has an electron arrangement of 2,8,1
This means that sodium needs to lose one electron to achieve a full outer shell.
The valency sodium is therefore 1 You learned in the 'Structure of the atom' topic, that atoms in the same group of the periodic table have similar chemical properties because they have the same number of electrons in their outer energy level. This means that they need to lose/gain the same number of electrons as each other to achieve a stable elecron arrangement. This means that they will have the same valency number as each other. These are as follows :
Group Some elements from group Valency
1 H, Na, Li 1
2 Be, Mg 2
3 B, Al 3
4 C, Si 4
5 N, P 3
6 O, S 2
7 F, Cl 1
0 He, Ne, Ar 0
Covalent bonding and valency
When two atoms combine, either by a covalent bond or an ionic bond, the ratio of atoms in compound is determined by the combining power or valency of each atom.
The valency for non-metal atoms tells us the number of covalent bonds that an atom needs to form to achieve a stable electron arrangement.
Valency card pictures help you decide how many covalent bonds need to be made, some of which are shown below :
For example, hydrogen atoms (group 1), need a further electron in their outer shell to achieve a stable electron arrangement, and therefore have a valency of 1. This means that each hydrogen atom needs to make one covalent bond with another non-metal atom. It is possible for two hydrogen atoms to form a single covalent bond together, forming a diatomic molecule of hydrogen as shown below.
Hydrogen atoms Hydrogen molecule For example nitrogen (group 5, electron arrangement : 2,5 and valency=3), needs to form three covalent bonds to achieve a stable electron arrangement. It can do this with three hydrogen atoms as shown below :
Nitrogen hydride Nitrogen atom Hydrogen atoms molecule
This method also works for other covalent compounds and diatomic molecules, as shown below :
The chemical formula of a covalent compound can be simplified by using the following technique :
1. Write the name of the compound 2. Write the symbols of the two atoms that are forming the covalent bond 3. Write the valency of the two atoms 4. Swap the valency of the two atoms over 5. Express this as the simplest ratio 6. The number under each tells you the number of each atom in the compound and hence, the chemical formula
The table below shows some examples of this method :
Hydrogen Hydrogen Carbon Carbon Phosphorus molecule oxide hydride oxide oxide Symbols H H H O C H C O P O Valency 1 1 1 2 4 1 4 2 3 2
Cross over 1 1 2 1 1 4 2 4 2 3 Simplest 1 1 2 1 1 4 1 2 2 3 ratio
Formula HH or H2 H2O CH4 CO2 P2O3
Ionic bonding and valency
When writing chemical formulae for ionic compounds, the valency method above also holds true, with the exception that the first three rows of group 4 of the periodic table do not usually form ions, and so cannot be given an ionic valency. When an ionic bond is formed between two atoms, a metal loses one or more electrons and a non- metal gains one or more electrons in order to give both atoms a stable electron arrangement. When this happens, two ions are formed - one with a positive charge (normally a metal) and one with a negative charge (a non-metal).
In order to form an ionic compound, the compound must have no overall charge associated with it. In other words there must be the same amount of positive charges present as negative charges. In this way, the chemical formula for an ionic compound can be deduced. This is shown below alongside the valency method :
Balancing Charges Valency method Sodium Magnesium Aluminium Sodium Magnesium Aluminium chloride sulphide oxide chloride sulphide oxide Symbol Na Cl Mg S Al O Symbol Na Cl Mg S Al O Charge Valency 1 1 2 2 3 2 on ion 1+ 1- 2+ 2- 3+ 2- formed Cross over No. of 1 1 2 2 2 3 each ion Simplest required 1 1 1 1 2 3 1 1 1 1 2 3 ratio to Chemical balance NaCl MgS Al O formula 2 3 charges Chemical NaCl MgS Al O formula 2 3
Often, ions exist that are not single charged atoms, but several atoms that are bonded together with an overall charge associated with them. The ions are called group ions and two examples are the sulphate ion and the nitrate (others can be found in the data booklet). 2- - The formula of these ions are SO4 and NO3 respectively.
The charge associated with these group ions can be classed as the valency when working out chemical formula.
e.g. The compound sodium sulphate would have the formula Na2SO4 and the compound calcium
nitrate would have the formula Ca(NO3)2.
Notice that you must put brackets around group ions if there is more than one group of them present in a chemical formula.
Determining chemical formulae from the chemical name
Often compounds exist that do not follow the simple valency rule for working out the chemical formula. The names of these compounds usually give an indication of the elements present and the quantity of each in the compound.
Carbon monoxide and carbon dioxide each contain carbon joined to oxygen. The prefixes 'mon' (or mono) and 'di' are used to show how many atoms of oxygen are present. The table shows show what the prefixes mean :
Prefix Meaning Mono or mon 1 Di 2 Tri 3 Tetra 4 Penta 5 Hexa 6
Carbon monoxide means carbon and one atom of oxygen Carbon dioxide means carbon and two atoms of oxygen
(No valency numbers are used to write formulae with prefixes.)
Another type of specially named compound involves elements that can have more than one valency number. This is true of many of the transistion metals.
These elements have their valency given as Roman numerals after the symbol for that element.
e.g. Copper (II) Chloride tells us that the element copper has the valency of 2 associated with it. This
would make the chemical formula CuCl2.
e.g. Copper (I) Chloride tells us that the element copper has the valency of 1 associated with it. This would make the chemical formula CuCl. Roman numeral : I II III IV V VI The commonly used roman numerals for valencies are : Valency : 1 2 3 4 5 6 The roman numeral after an element also tells you the size of the charge that exist on that atom.
Balanced equations
When a reaction occurs, we can write a word equation that explains the reaction that has taken place. You learned how to do this in the 'Substances' topic at the beginning of this unit. Remember that the reactants are written on the left of the arrow (the beginning of the reaction) and the products are written on the right of the arrow (the end of the reaction).
e.g. When magnesium metal was burned in air (a source of oxygen), the reaction gave a bright glow and a new compound called magnesium oxide was formed.
The word equation for this reaction is :
magnesium + oxygen magnesium oxide
Since you have now learned how to write the chemical formula of compounds and elements, you can replace these words with the chemical formulae of each of the substances.
The element magnesium is written as Mg
The element oxygen (a diatomic molecule) is written as O2 The compound magnesium oxide is written as MgO (both magnesium and oxygen have a valency of two and when these valencies are 'crossed over' and expressed as the simplest ratio, the formula gives MgO).
The equation now looks like this : Mg + O2 MgO
This equation is called an unbalanced equation, as the number of each type of atom on the left of the arrow is not the same as the number of each type of atom on the right of the arrow. In this example, the amount of oxygen on the left hand side of the arrow is not the same as the amount of oxygen on the right hand side of the arrow. We need to form 2 'lots' of MgO so as to have two oxygens on the right hand side of the equation.
The equation now looks like this :
Mg + O2 2MgO
Again this equation is still not entirely accurate as we now have two magnesium on the right hand side of the arrow, but only one on the left hand side . The reaction must need another magnesium at the beginning of the reaction (on the left hand side of the arrow).
The equation now looks like this :
2Mg + O2 2MgO
This is what we call a balanced equation. i.e. the number of atoms of each element on the left hand side of the arrow is the same as the number of atoms of each element on the right hand side of the arrow.
In general, to write a balanced chemical equation :
1. Write a word equation 2. Put the chemical formula for each reactant and product in the reaction (remembering about diatomic elements) 3. Count the number of each type of atom on each side of arrow 4. Multiply the necessary formulae to balance each type of atom 5. Repeat the previous 2 steps until the number of each type of atom on each side of the arrow is the same
Formula mass
The formula mass of an element or compound is the sum of the mass of all of the atoms that it contains.
In the 'Structure of the atom' topic, you learned that atoms in the same element can have different masses due to different numbers of neutrons and these atoms were called isotopes. When we work out the formula mass of an element or compound, we take into consideration the mass of the isotopes and the percentage of each. This is done by simply using the Relative Atomic Mass of the elements involved. These values are given in the data book.
In the element Helium (chemical formula - He), the formula mass is simply the relative atomic mass of one atom of helium. This is given in the data book as 4. Therefore the formula mass of Helium is four.
If we consider the element Hydrogen, we must remember that it is a diatomic element and the formula mass is therefore 2 x 1 = 2. Another example is the molecule Hydrogen chloride. This has one atom of hydrogen (relative atomic mass = 1) and one atom of chlorine (relative atomic mass of 35.5). This give the formula mass of hydrogen chloride as 36.5
Work out the formula mass of the compound ammonium phosphate.
Firstly write the chemical formula. ammonium phosphate + 3- Symbols : NH4 PO4 Valency : 1 3
Cross over valencies : 3 1 Simplest ratio : 3 1
Formula : (NH4)3PO4
Next work add up the relative atomic masses of all of the elements involved. 3 x N = 3 x 14 = 42 12 x H = 12 x 1 = 12 1 x P = 1 x 31 = 31 4 x O = 4 x 16 = 64 Formula mass = 149
The formula mass of the compound ammonium phosphate is 149.
The mole
One mole of a substance is equal to the formula mass expressed in grams. It is also called the gram formula mass.
In the previous worked example for the compound ammonium phosphate, the formula mass was calculated to be 149. This means that one mole of ammonium phosphate has the mass of 149g.
The units for the mole is mol.
When working out calculations involving formula masses and moles, you can use the triangle on the right.
What is the mass of 2 moles of hydrogen chloride ?
Firstly write the chemical formula.
Symbols : H Cl Valency : 1 1
Cross over valencies : 1 1 Simplest ratio : 1 1 Formula : HCl
Next work add up the relative atomic masses of all of the elements involved. 1 x H = 1 x 1 = 1 1 x Cl = 1 x 35.5 = 35.5 Formula mass = 36.5
The formula mass of the compound hydrogen chloride is 36.5.
This means that one mole of hydrogen chloride has a mass of 36.5g Therefore two moles of hydrogen chloride has a mass of (2 x 36.5) = 73g
How many moles of Copper (II) Carbonate are there in 494g of the substance ?.
Firstly write the chemical formula.
2+ 2- Symbols : Cu CO3 Valency : 2 2
Cross over valencies : 1 1 Simplest ratio : 1 1
Formula : CuCO3
Next work add up the relative atomic masses of all of the elements involved. 1 x Cu = 1 x 63.5 = 63.5 1 x C = 1 x 12 = 12 3 x O = 1 x 16 = 48 Formula mass = 123.5
The formula mass of the compound copper (II) carbonate is 123.5.
This means that one mole of copper (II) carbonate has a mass of 123.5g Therefore 494g contains (494/123.5) = 4 moles of copper (II) carbonate (or 4 mol).
NEW WORDS AND THEIR MEANINGS
VALENCY / COMBINING POWER - Gives information about how many single bonds an atom (or group of atoms) needs to form to achieve a stable electron arrangement. It is also the number of electrons that an atom needs to lose or gain in order to achieve a stable electron arrangement.
+ GROUP IONS - A group of atoms that have an overall charge (e.g. the ammonium ion - NH4 ). These ions are listed in the data booklet.
WORD EQUATION - A way of representing a chemical reaction using the names of the reactants and the products.
UNBALANCED EQUATION - A way of representing a chemical reaction using the chemical formulae of the reactants and the products.
BALANCED EQUATION - This is obtained when the numbers of each type of atom on the left a reaction arrow (the reactants) is the same as the number of each type of atom on the right of the reaction arrow (products) when chemical formulae are written. FORMULA MASS - The sum of the relative atomic masses of all the atoms in an element or compound.
MOLE - The formula mass of a substance expressed in grams.
GRAM FORMULA MASS - The formula mass of a substance expressed in grams (another term for 1 mole of a substance).
Unit 2 Carbon Compounds
What are fuels?
Fuels are chemicals which burn, giving out energy. The reaction is described as exothermic (energy releasing), and the gas oxygen is used up in the process.
Combustion
A reaction in which oxygen is used up as energy is released is known as a combustion reaction. This is another word for the process of burning something.
The combustion of carbon to form carbon dioxide
Hydrocarbons
Many of the fuels that we use are fossil fuels that were formed millions of years ago from material that was once living. There are three common fossil fuels - Coal, oil and natural gas
The chemicals that mostly make up oil and natural gas are called hydrocarbons. They are given this name because these compounds are made up using the elements hydrogen and carbon only.
When hydrocarbons burn in a plentiful supply of oxygen, the carbon 'part' of the hydrocarbon effectively joins with the oxygen to form carbon dioxide and the hydrogen 'part' of the hydrocarbon effectively joins with the oxygen to form hydrogen oxide, which is better known as water.
When this combustion takes place in a plentiful supply of oxygen, we call it complete combustion. This can be summarised by using a general word equation for all hydrocarbons:
hydrocarbon oxygen carbon dioxide water
The carbon dioxide that is formed during the combustion of a hydrocarbon can be tested with lime water. When carbon dioxide is passed through lime water, it changes the lime water from colourless to 'milky' white. The water that is formed can be tested by measuring the freezing point and boiling point and comparing it to that of pure water, or it can be tested by using cobalt chloride paper which turns from blue to pink in the presence of water. The fact that water (hydrogen oxide) is produced proves that the fuel contains hydrogen. The fact that carbon dioxide is produced proves that the fuel contains carbon.
The products of burning a hydrocarbon can be identified as follows:
Pollution problems with burning hydrocarbons
When we burn hydrocarbons in a plentiful supply of oxygen, we produce water and carbon dioxide.
Carbon dioxide is a gas which causes global warming (greenhouse effect).
The main pollution problems associated with the combustion of hydrocarbons occur when the fuel is burned in a poor or limited supply of oxygen. This is called incomplete combustion. When this happens carbon moxoxide gas is produced in addition to particles of carbon (soot).
Carbon monoxide is a poisonous gas that permanently attaches to the red blood cells in our body preventing the transfer of oxygen in our bloodstream. Soot particles (carbon) are harmful when breathed in as they can be deposited in our lungs, which can eventually make breathing more difficult.
When we burn fossil fuels (which are mainly hydrocarbons), there is also a small amount of sulphur compounds present. This sulphur, on combustion with oxygen, forms sulphur dioxide (a poisonous gas) that can dissolve in the rain to form sulphurous acid - also known as acid rain.
Sulphur dioxide can also trigger asthma attacks and it is often removed from fuels before they are burned so that pollution is reduced.
Fuels need oxygen from the air to burn. In petrol engines, the spark provides the activation energy to ignite the fuel. Unfortunately the spark can also cause nitrogen and oxygen molecule to combine to make nitrogen oxides.
These are poisonous gases which add to the acid rain problem. The same gases are formed when a lightning bolt passes through the air.
Reducing pollution from car engines
A special exhaust system called a catalytic converter was introduced in the UK in 1991. This reduced the quantities of harmful gases such as nitrogen oxide, carbon monoxide and unburnt hydrocarbons, by changing them into less harmful gases such as nitrogen and carbon dioxide.
Engines which use more air and less fuel also help prevent the formation of carbon monoxide as the fuel has sufficient oxygen to be completely.
Fractional Distillation
Crude oil is a mixture of many compounds which are similar to each other in chemical makeup and chemical properties, but differ in size of molecule. As a result the different molecules have different melting and boiling points.
Distillation will separate two liquids that are mixed together if they have different boiling points.
When there are more than two substances present, process called fractional distillation is used.
The term 'fractional' refers to parts obtained by splitting the crude oil mixture.
Fractions are groups of hydrocarbons that have similar boiling points and so are collected at the same position of the fractionating tower. The fraction with the lowest boiling point evaporates first and can be separated from fractions with higher boiling points.
This is done in the oil industry using very tall towers called fractionating towers as shown in the photograph.
Fractions with the lowest boiling point are collected higher up the tower.
Properties of the fractions
The fractions with the lowest boiling point (first fractions obtained) are pale liquids while as the boiling point increases the fractions get darker in colour.
The residue contains molecules with boiling points higher than 350°C and is a black solid called bitumen that is used to cover road surfaces (road tar).
The table below shows how the properties flammability, evaporation and viscosity change as the boiling point of the fraction increase.
The bigger the molecules, the higher the boiling point, the lower the flammability and the greater the viscosity. Big molecules are more strongly attracted to each other. Higher temperatures are needed to separate the molecules when they are boiled. Big molecules don't react as quickly with oxygen and so are less flammable. The stronger attractions between the bigger molecules means that liquids containing bigger molecules don't flow as easily as liquids with smaller molecules. Longer molecules also tangle together more and are more viscous.
NEW WORDS AND THEIR MEANINGS
FUEL - a substance which burns releasing energy
EXOTHERMIC - a reaction in which energy is released
COMBUSTION - a reaction of a substance with oxygen, giving out energy
FOSSIL FUELS - fuels such as coal and oil that were formed millions of years ago by the decompostion of material that was once living (such as trees and animals)
HYDROCARBONS - compounds containing carbon and hydrogen only
COMPLETE COMBUSTION - when there is sufficient oxygen for a fuel to burn completely.
LIME WATER - a solution of calcium hydroxide that turns milky in the presence of carbon dioxide. This is the test for carbon dioxide.
COBALT CHLORIDE PAPER - This paper can be used to test for water. It turns from blue to pink when water is present.
POLLUTION - substances which damage the environment
INCOMPLETE COMBUSTION - when there is insufficient oxygen for a fuel to burn completely.
ACID RAIN - when gases such as sulphur dioxide and carbon dioxide dissolve in water in the clouds, they form acids (sulphurous acid and carbonic acid), which can cause damage to the environment..
CATALYTIC CONVERTER - a device on cars, containing platinum, to reduce harmful gases such as carbon monoxide, nitrogen oxides and unburnt hydrocarbons.
FRACTIONAL DISTILLATION - separation of a mixture of substances into parts or fractions based the fact that they have different boiling points FRACTION - a mixture of substances that have similar boiling points and are collected together during fractional distillation.
FLAMMABILITY - refers to how easily a substance burns e.g. a flammable substance burns easily
VISCOSITY - describes the thickness of a liquid e.g. a viscous liquid does not run easily.
Nomenclature, structural formulae and Isomers
Hydrocarbons
a) Alkanes, CnH2n+2
There are several sets of hydrocarbons, and the alkanes make a sub-set. Alkanes all have names which end in the letters -ANE.
The Chemistry data book (page 6) gives the names of the first 8 members of the sub-set.
Methane is the simplest member of the alkane sub-set and has the formula CH4, while the second member of the subset, is called ethane and has the formula C2H6.
In methane there are four hydrogen atoms join to one atom of carbon.
Formulae like this (CH4 and C2H6) are called molecular formulae.
The arrangements of atoms in methane, ethane, propane and butane are shown below.
These formulae below show exactly how the atoms are joined and are called full structural formulae.
There is a pattern in the formula as shown in the diagram below:
All members of the alkane family fit the general formula CnH2n+2.
This can be used to work out the formula of any alkane if the number of carbon atoms is known. CnH2n+2 ane
Shortened structural formulae can also be drawn for alkanes (and other sub-sets).
Shortened structural formulae only show the numbers of hydrogen atoms associated with each carbon atom.
A comparison of molecular and shortened structural formulae of the alkane sub-set is shown below:
Alkane Molecular Formula Shortened Structure
Methane CH4 CH4
Ethane C2H6 CH3-CH3
Propane C3H8 CH3-CH2-CH3
Butane C4H10 CH3-CH2-CH2-CH3 or CH3-(CH2)2-CH3
Pentane C5H12 CH3-(CH2)3-CH3
Hexane C6H14 CH3-(CH2)4-CH3
Heptane C7H16 CH3-(CH2)5-CH3
Octane C8H18 CH3-(CH2)6-CH3
In this table, the name octane refers to the alkane in which 8 carbon atoms are joined in a line with 18 hydrogen atoms attached. Heptane refers to the alkane with 7 carbon atoms joined in a line etc.
It is possible to have chains with branches on them.
The diagram below shows two different structures for the alkane of molecular formula C4H10.
These different structures are known as isomers and are defined as compounds with the same molecular formula but with a different structural formula.
Rules for naming branched-chain alkanes
The branches are based on alkanes and have the general formula CnH2n+1
The branches or groups of atoms are called alkyl groups and are named by replacing the -ANE
ending of the alkane with -YL e.g CH3 = methyl group, C2H5 = ethyl group etc
Method for naming
1. Look for the longest continuous chain of carbon atoms and name this as the parent compound. 2. The parent name is placed at the end of the name of the compound. 3. Look for groups which form branches on the chain and identify them. 4. Different groups are written in alphabetical order before the parent name e.g. 'ethyl' before 'methyl'. 5. If there are 2 identical groups the prefix 'di' is placed before the name of the branch e.g. 'dimethyl', 'diethyl' etc 6. The prefixes 'tri' and 'tetra' are used if there are 3 or 4 repetitions respectively of the same group on the parent chain. 7. To specify the position of each group, the parent chain is numbered from the end which results in the lowest possible numbers in the formula.
The two isomers shown above are called butane and methylpropane. No number is needed in the name methylpropane because the only possible position for the methyl branch is on the middle carbon atom of propane.
The different structures for C6H14 are shown below: Full structure A is hexane. Structures B and C have both have 5 carbon atoms in a chain and because of this they are said to be based on pentane. However, they are different to pentane because they each have a CH3 group in place of a hydrogen atom.
Compounds B and C are both called methylpentane, but are different in the position of the methyl groups on the pentane chain. To distinguish them, the pentane chain is numbered from the end that gives the lowest number in the name.
Numbering from the right names B as 2-methylpentane - the correct name. (Numbering from the left would give 4-methylpentane - the incorrect name.
Compound C is called 3-methylpentane (and it doesn't matter which end the numbering starts).
Compounds D and E are based on butane with two methyl groups. The number of methyl groups is indicated using the prefix 'di-' in the name, and the positions of the methyl group indicated by separate numbers.
D is correctly named as 2,3-dimethylbutane, while E is 2,2- dimethylbutane.
Representing shortened branched structural formulae
Shortened structural formulae are written in the usual way and side chains are included in the formula by writing them in brackets after the carbon they are attached to. Compound B has the formula CH3CH2CH2CH(CH3)CH3 while
compound C has the formula CH3CH2CH(CH3)CH2CH3
Compound D has the formula
CH3CH(CH3)CH(CH3) CH3 while compound E has the formula
CH3C(CH3)2CH2CH3
Nomenclature is a special term which means 'naming'.
The alkanes make a collection called a homologous series because all alkanes fit the same general formula, have similar chemical properties and show a gradual but regular change in physical properties such as melting and boiling points.
b) Alkenes, CnH2n
Alkenes form another homologous series of hydrocarbons with names ending in the letters -ENE.
Alkenes have a double bond between 2 carbon atoms, and this is the reason for two fewer hydrogen atoms than alkanes.
CnH2n ene
The table gives the names, molecular formulae and shortened structure for the first 7 members of the alkene series.
Alkene Molecular Formula Shortened Structure
Ethene C2H4 CH2=CH2
Propene C3H6 CH3-CH=CH2
Butene C4H8 CH3-CH2-CH=CH2
Pentene C5H10 CH3-(CH2)2-CH=CH2
Hexene C6H12 CH3-(CH2)3-CH=CH2
Heptene C7H14 CH3-(CH2)4-CH=CH2
Octene C8H16 CH3-(CH2)5-CH=CH2
Isomers exist for alkenes by using branches, but the presence of the double bond also increases the possibility of isomers because the position of the double bond can vary.
When naming alkenes, the position of the double bond is indicated by numbering the carbon chain from the end that gives the lowest number for the double bond. Although the double bond is between 2 carbon atoms, only the number of the lowest carbon atom is included in the name.
In the carbon skeleton C-C-C=C, the double bond is between carbon atoms 1 and 2, and so the chain is called but-1-ene.
In the carbon skeleton C-C=C-C, the double bond is between carbon atoms 2 and 3, and so the chain is called but-2-ene.
Some examples of names, full structural formulae and shortened structural formulae are given below.
c) Cycloalkanes, CnH2n
Cycloalkanes form another sub-set of hydrocarbons.
Their names are related to the alkane sub-set by ending in the letters -ANE, and they can be identified as cycloalkanes because they begin 'CYCLO-'
They have only single bonds between carbon atoms (like alkanes), but do no exist as chains. Instead, one end of an alkane chain has joined to the other end of the alkane chain to make a 'cyclic' (or ring) structure. To allow this to happen, one hydrogen atom from each end of the alkane chain has to be removed to allow the chain to form a 'ring' structure.
The general formula for cycloalkanes is CnH2n (the same as for the alkene sub-set). CnH2n cyclo ane
The smallest cycloalkane is cyclopropane (C3H6), and continues with cyclobutane (C4H8) etc.
The shortened structural formulae for several cycloalkanes are shown below:
Remember that cycloalkanes have the same general formula as alkenes and so some isomers can belong to a different homologous series e.g propene and cyclopropane are isomers.
Alkanols and Alkanoic Acids
Alkanols, CnH2n+1OH
Alkanols make a homologous series of compounds based on the Alkanes. The ending -OL indicates membership of the family, and the presence of the hydroxyl group, -OH instead of one of the hydrogen atoms.
CnH2n+1OH anol
Alkanol Molecular Formula Shortened Structure
Methanol CH3OH CH3-OH Ethanol C2H5OH CH3-CH2-OH
Propanol C3H7OH CH3-CH2-CH2-OH
Butanol C4H9OH CH3-CH2-CH2-CH2-OH or CH3-(CH2)3-OH
Pentanol C5H11OH CH3-(CH2)4-OH
Hexanol C6H13OH CH3-(CH2)5-OH
Heptanol C7H15OH CH3-(CH2)6-OH
Octanol C8H17OH CH3-(CH2)7-OH
Isomers exist for alkanols, and the presence of the -OH or hydroxyl group increases the possibility of isomers because the position of the hydroxyl group can vary.
When naming alkanols, the position of the hydroxyl group on the carbon chain longest chain is numbered so that the hydroxyl group in attached to the lowest numbered carbon atom.
The shortened formula for propan-1-ol is CH3-CH2-CH2-OH
Alkanoic Acids
Alkanoic acids have names which end in -OIC and contain the carboxyl group, -COOH.
Cn-1H2(n-1)+1CO anoic acid
Alkanoic acid Molecular Formula Shortened Structure Methanoic acid HCOOH H-COOH
Ethanoic acid CH3COOH CH3-COOH
Propanoic acid C2H5COOH CH3-CH2-COOH
Butanoic acid C3H7COOH CH3-CH2-CH2-COOH or CH3-(CH2)2-COOH
Pentanoic acid C4H9COOH CH3-(CH2)3-COOH Hexanoic acid C5H11COOH CH3-(CH2)4-COOH
Heptanoic acid C6H13COOH CH3-(CH2)5-COOH
Octanoic acid C7H15COOH CH3-(CH2)6-COOH
Esters
Esters contain the ester linkage, -COO- and can be recognised because their names end in the letters -OATE. Esters are made when an alkanol and an an alkanoic acid join together e.g. methyl ethanoate is made when methanol joins with ethanoic acid.
Alkanol Alkanoic acid Name of ester Methanol Methanoic acid Methyl methanoate Ethanol Methanoic acid Ethyl methanoate Methanol Ethanoic acid Methyl ethanoate Ethanol Ethanoic acid Ethyl ethanoate Methanol Propanoic acid Methyl propanoate Ethanol Propanoic acid Ethyl propanoate
To name an ester replace the 'ANOL' ending of the alkanol with 'YL'; replace the 'OIC ACID' ending of the alkanoic acid with 'OATE' and put the two names together.
The shortened structural formula for methyl ethanoate is CH3-COO-CH3
NEW WORDS AND THEIR MEANINGS
HYDROCARBON - compounds which contain the elements hydrogen and carbon only.
MOLECULAR FORMULAE - formulae which show the elements present, and the number of atoms of each element.
FULL STRUCTURAL FORMULAE - formulae which show how the different elements present are joined together.
GENERAL FORMULA - a formula which shows the ratio of different atoms applicable to all members of the homologous series.
SHORTENED STRUCTURAL FORMULAE - structures in between molecular formulae and full structural formula. They show the number of hydrogen atoms joined to each carbon atom without showing how the atoms are joined together.
ISOMERS - compounds with the same molecular formula but with a different structural formula.
HOMOLOGOUS SERIES - family of compounds with similar chemical properties, a gradation in physical properties and where all members of the family fit the same general formula.
Reactions of carbon compounds
(i) Addition
Alkanes and cycloalkanes are saturated compounds and this means that they contain only carbon to carbon single bonds.
Saturated hydrocarbons such as these cannot undergo addition reactions.
Alkenes are unsaturated compounds and these contain at least one carbon to carbon double bond.
Alkenes undergo addition reactions with molecules such as hydrogen, H2, the Halogens and Water.
The double bond is broken and replaced by a single bond causing the unsaturated alkene to become a saturated product.
The rapid decolourisation reaction with bromine is used as the test for an alkene and distinguishes it from an alkane.
Water can also add to an alkene and the addition is called hydration. The reaction of ethene with water in the presence of phosphoric acid as a catalyst (catalytic hydration) is an industrial method of making alcohol, and supplements the alcohol made by fermentation.
(ii) - Cracking
Cracking of long-chain alkanes
Crude oil contains more long chain fractions than are needed and not enough short chain fractions to meet the demand for fuels. A process called cracking is used to break long chains into smaller molecules which are more useful. High temperatures cause bonds to break in the long chain alkane and a mixture of compounds, some of which are unsaturated, is formed. The presence of unsaturated hydrocarbons can be shown by testing with bromine water.
The equation shows how an alkane molecule can be cracked into a shorter alkane and an alkene. As cracking occurs using heat, the process is known as thermal cracking. Cracking cannot give two saturated products as there are not enough atoms of hydrogen to allow both products to be saturated.
The cracking can be carried out at a lower temperature if a catalyst made from aluminium oxide or aluminium silicate is used.
The process is called catalytic cracking and is preferred in the oil industry because it saves energy (and money). (iii) Ethanol
Ethanol is the alcohol in alcoholic drinks, and it is made by a reaction called fermentation of glucose using an enzyme in yeast to catalyse the reaction.
Glucose ------> alcohol + carbon dioxide + energy
As the ethanol concentration rises during fermentation, the yeast is killed and this limits the alcohol concentration from fermentation to about 15%.
Higher concentrations can be obtained by distillation, and the drink obtained is called a 'spirit' drink.
Whisky, vodka, brandy and gin are examples of spirits and have alcohol concentrations of up to 40%.
Effect of Alcohol on the Body and Mind
Alcohol affects the brain and increases reaction time. This means that it will take longer to react to situations making accidents more likely. Alcohol can affect speech and steadiness. Excessive alcohol consumption can cause liver damage and cancer of the mouth/throat.
Other Ways of making Alcohol
Hydration of ethene produces ethanol as described in 'Addition reactions' earlier.
It is possible to convert the ethanol back into ethene by removing the water in a dehydration reaction.
The dehydration reaction can be achieved by passing alkanol vapour over heated aluminium oxide. The double bond is produced by the loss of the hydroxyl group and a hydrogen atom from an adjacent carbon atom.
Dehydration of ethanol will produce ethene.
Ethanol as a Fuel
Ethanol is a fuel because it burns releasing energy.
C2H5OH(l) + 3O2 ------> 2CO2(g) + 3H2O(l)
Ethanol can be mixed with petrol and used as a fuel for cars. As ethanol can me made by fermenting sugar cane, and sugar cane can be grown annually, ethanol is regarded as a renewable fuel.
(iv) - Making and Breaking Esters
Making Esters
Esters are made by a condensation reaction between carboxylic acid and an alcohol in which a molecule of water is eliminated from the functional groups of the carboxylic acid and alcohol. The -COO- group in the ester is called the ester link and it is formed between the hydroxyl group of the alcohol and the carboxyl group of the carboxylic acid.
Esters are made by warming the carboxylic acid and alcohol in a test tube containing a few drops of concentrated sulphuric acid and heated by a water bath for about 10 minutes. This prevents the reaction mixture catching fire. However, to prevent the reactants and products being lost during heating, a wet paper towel is wrapped around the outer, upper part of the test tube. This causes volatile reagents to condense and run back into the test tube in a technique called refluxing
The process is reversible i.e it operates in both directions. Sulphuric acid is a catalyst for the reaction and as a dehydrating agent, it removes the water that is formed. By removing the water from the reaction, the reverse reaction is prevented, so that more ester is made.
The ester is obtained by pouring the mixture into a beaker containing an aqueous solution of sodium hydrogencarbonate to neutralise the sulphuric acid.
Evidence that an ester is formed is its typical smell, and that is appears as a solid/oily liquid on the water.
Breaking Down Esters
When an ester is heated with water it begin to break down into a carboxylic acid and alcohol. This breaking down reaction with water is called hydrolysis and it is the exact opposite of condensation.
It is not possible by this method to totally break down all of the ester and the reaction mixture will contain some ester, water, carboxylic acid and alcohol.
The reaction by be speeded up using H+ ions or OH- ions. If an alkali is used, this further helps by reacting with the carboxylic formed in the hydrolysis and removing the carboxylic acid from the reaction. This prevents the joining of the alcohol with the carboxylic acid to remake the ester.
NEW WORDS AND THEIR MEANINGS
SATURATED - compounds that contain only carbon to carbon single bonds eg alkanes and cycloalkanes.
UNSATURATED - compounds that contain at least one carbon-carbon double bond eg alkenes.
ADDITION - a reaction where a small molecule adds onto an unsaturated substance causing it to become more saturated.
HYDRATION - an addition reaction where water is added to an unsaturated substance.
DEHYDRATION - a reaction in which water is removed from a substance.
CONDENSATION REACTION - a reaction where two or more molecules join together and a small molecule, usually water is eliminated.
REFLUXING - where liquid evaporate to form gases which are then re-condensed to continue reaction.
HYDROLYSIS - a reaction in which a molecule is broken into smaller pieces using water.
Origin of plastics
Crude oil is used to make most plastics and synthetic fibres.
Long chain hydrocarbons can be cracked or broken into a mixture of short chain hydrocarbons, some of which are saturated and others unsaturated.
Synthetic fibres
Synthetic fibres are man-made (made by the chemical industry) and do not occur naturally. Plastics are examples of synthetic materials.
Many items of clothing contain materials such as polyester, polyamide, terylene, rayon, dralon etc. which are all man-made fibres, and are called synthetic fibres.
Properties of plastics in relation to their uses
Plastics Uses Properties Poly(ethene) Plastic bags Strong, light, waterproof Poly(ethenol) Surgical stitching material Water soluble Polyamide Blouses, tights Hard wearing Wears well, helps keep shape of Polyester Shirts, blouses, quilts clothing, warm Polystyrene Drinking cups, packaging Light, heat insulator Polyvinyl chloride Coating on electrical wires, drain-pipes Electrical insulator, flexible (PVC) Does not melt or conduct heat or Bakelite Pot handles, electrical sockets electricity Dralon Furniture covers Hard wearing, stain resistant Bullet proof clothing, puncture resistant Kevlar Very strong fuel tanks Rubber Soles of shoes, tyres Flexible, waterproof
Advantages and disadvantages of plastics
Plastic Advantages Disadvantages Polyester Strong, light Not as warm as wool Poly(ethene) Cheap, waterproof Does not rot away Melamine/Formica Heat resistant Not as attractive as wood Polyurethane foam Makes cheap seating Gives toxic fumes when burned Polystyrene Makes shaped, colourful TV cases Not as attractive as wood
Problems when plastics burn
Some plastics burn or smoulder and give off toxic fumes. They can produce thick black smoke, make toxic fumes and use up large amounts of oxygen.
As plastics contain carbon, they all produce carbon dioxide, carbon monoxide (as air runs out), and smoke.
Plastic Elements present Toxic fumes Poly(ethene) Carbon and hydrogen Carbon monoxide P.V.C. Carbon, hydrogen and chlorine Hydrogen chloride (acidic) Polyurethane foam Carbon, hydrogen and nitrogen Hydrogen cyanide (toxic) Polystyrene Carbon and hydrogen Dense, black smoke
Biodegradable Plastics
Plastic packaging does not rot away and causes major litter problems. Many plastics are non- biodegradable, meaning they will not rot away in nature.
Plastics are now being introduced that are biodegradable e.g. Biopol (biodegradable polymer).
Thermoplastic and thermosetting polymers
Thermoplastic plastics are those which can be resoftened on heating e.g. poly(ethene), poly(amide).
Thermosetting plastics are those which cannot be resoftened on heating e.g. bakelite, melamine.
Both type of plastics consist of long, tangled chains but in thermosetting polymers, there are links between the chains which gives a much more rigid structure.
Plastics are polymers
A polymer is a very big molecule made from many small molecules (called monomers) which repeat through the structure.
The process in which the monomers join to make a polymer is called polymerisation.
Structure of the monomers
Many plastics or polymers are made from unsaturated monomers obtained by cracking fractions of crude oil.
The simplest monomer is ethene, C2H4, an alkene (which contains a carbon to carbon double bond).
Ethene monomers join together to give a polymer called poly(ethene), which is often called polythene.
This process is called polymerisation
How do monomers join to make a polymer?
1. Addition Polymerisation
A chemical is added which breaks the double bond between the two carbon atoms of ethene to make a very reactive unit.
Reactive units join together, end to end and a big molecule or polymer is made.
This molecule consists of many small units joined together and repeating along the length of the polymer.
The process is called addition polymerisation because the monomer units join (or add) together to give one product, by a series of reactions in which the double bond breaks.
Many plastics are made from alkenes, or from unsaturated molecules made from alkenes.
Modern Name of Original Name of Modern Polymer Original Polymer Monomer Monomer Name Name Ethene Ethene Poly(ethene) Polythene Polyvinyl chloride Chloroethene Vinyl chloride Poly(chloroethene) (P.V.C.) Propene Propene Poly(propene) Polypropene Phenylethene Styrene Poly(phenylethene) Polystyrene
2. Condensation polymerisation o When monomer units join together, they can do so not only by breaking double bonds, but also by reacting across functional groups between the monomers. o When monomers have two functional groups in each molecule, a long polymer chain can be built up by reacting the functional groups at either end of the molecule. o When these functional groups react together, a new bond is formed between the monomer units and a small molecule (often water) is released. When this happens, the reaction is described as condensation polymerisation.
An example of a condensation reaction was covered in the Nomeclature topic and the Reaction of carbon compounds topic during the formation of an ester.
An example of a condensation polymerisation reaction is shown to the right.
When the polymer forms between monomers that contain two functional groups (the -OH and the -COOH groups), a water molecule is released from the compound, allowing a new bond to form between the monomer groups.
Polyesters
Polyesters are condensation polymers in which the monomers units are linked together by ester groups (-COO-). This happens when monomer units combine to form an ester linking group (as in the above reaction).
In the reaction shown on the right, an alcohol monomer with two -OH groups (one at either end of the molecule) reacts with a carboxylic acid monomer that has two -COOH groups (again, one at either end of the molecule).
Again, when the acid group (-COOH) reacts with the alcohol group (-OH), a new bond is formed and a molecule of water is released in the process. This means that when we form a polyester, the reaction is called a condensation polymerisation.
Amines
The amines belong to a homologous series based on the alkanes in which a hydrogen has been replaced with the amine functional group -NH2.
The names of the members of this series are derived by the prefix which tells you the number of carbon atoms present and all have the ending -amine. Some members of this family are shown below:
Shortened Structure Name Structural formula structural formula
Methylamine CH3NH2 CH5N
Ethylamine CH3CH2NH2 C2H7N
Propylamine CH3CH2CH2NH2 C3H9N
Butylamine CH3CH2CH2CH2NH2 C4H11N
Polyamides
Polyamides are polymers that are formed in a reaction between an amine and a carboxylic acid group in the monomer units. The monomer units join together and form an amide link (-CONH-).
Either the monomers have both a carboxylic acid group and an amine group present, or two monomers are required: One with two carboxylic acid groups and another with two amine groups present.
NEW WORDS AND THEIR MEANINGS PLASTICS - a wide variety of large molecules made from products from crude oil distillation
SYNTHETIC - man-made
FIBRE - a large molecule which is made into long threads
NATURAL - occurring in nature
BIODEGRADABLE - broken down into smaller pieces by living organisms
TOXIC - harmful
THERMOPLASTIC - a plastic that can be resoftened by heating e.g. poly(ethene)
THERMOSETTING - a plastic that cannot be resoftened by heating e.g. bakelite
MONOMERS - small units that join together to give a very big molecule
POLYMERS - very big units made when many monomer molecules join together
POLYMERISATION - the process in which monomers join to give a polymer
CRACKING - a reaction in which long-chain hydrocarbons are converted into unsaturated hydrocarbons
ADDITION POLYMERISATION - the making of a polymer by a series of addition reactions
FUNCTIONAL GROUPS - a groups of atoms in a compound that give the compound certain properties. e.g. the -OH group in alkanols and the -COOH group in alkanoic acids
CONDENSATION POLYMERISATION - Polymerisation that occurs between two functional groups and a small molecule is released (this is often water, but can also be another small molecule).
POLYESTERS - a condensation polymer in which the monomers are linked by ester groups (-COO-).
AMINE - a homologous series with the functional group -NH2.
POLYAMIDE - a condensation polymer which involves the reaction between an amine and a carboxylic acid group in the monomer units. The monomers then link using an amide link (-CONH-).
Carbohydrates
Plants use sunlight, carbon dioxide and water to make an important class of foods called carbohydrates in a process called photosynthesis.
The importance of Carbohydrates
Carbohydrates are an important food for animals. Respiration is important for all living things because it provides energy when glucose is burned or broken down in the body.
Glucose + oxygen ------> carbon dioxide + water + energy C6H12O6 + 6O2 ------> 6CO2 + 6H2O
Carbohydrates release energy when burned, and make carbon dioxide and water. The release of energy can be seen in the custard-powder tin experiment. Animals use this energy for many things such as movement, and warmth to keep our body temperature at 37°C.
What are carbohydrates?
Carbohydrates are compounds which contain the elements carbon, hydrogen and oxygen only. In these compounds the hydrogen and oxygen are in the ratio of two to one - in other words there is always twice as many hydrogen atoms as oxygen atoms.
There are actually two different types of carbohydrates: sugars and starches.
Sugars
Sugars are small carbohydrate molecules that can dissolve in water.
These small sugar molecules can be dissolved in our bloodstream so they can be carried to parts of the body that require energy.
Some examples of sugars are: glucose, fructose, maltose and sucrose (which is also known as table sugar).
C a r b o h F y o d r a t e G l u cC o6 s e F r u c C t o6 s e Maltose C12H22O11
Sucrose C12H22O11
Most sugars can be detected by the Benedict's test; sucrose is an exception. In this test, Benedict's solution turns from blue to brick-red in colour after heating with a sugar in a water bath. This is a positive test for sugars (except sucrose).
Starches
When molecules join together and water molecules are eliminated when they join, the reaction is called condensation.
If many glucose molecules join together and a polymer is made, the reaction is called condensation polymerisation.
Starch is a natural condensation polymer made of many glucose molecules linked together.
Carbo For hydrat mula e (C H Starch 6 1 0O5)n
Starch can be detected using the iodine test. In this test starch turns iodine solution form reddish- brown to blue-black.
Plants convert glucose into starch for storing energy.
To show that starch is made in leaves by photosynthesis
1. Put plant in dark for 2 days to remove all food from the leaf. 2. Put plant in the light for one day to let it make food. 3. Remove one leaf and boil in water to kill it. 4. Put in hot alcohol to remove chlorophyll - the leaf will be white. 5. Wash in water to remove alcohol and add iodine solution to it. 6. The leaf will be blue-black proving that starch had been made.
During digestion, the large starch molecules are hydrolysed (broken down by the action of water) into smaller glucose molecules that can then be transported around the body in our bloodstream. This reaction is the reverse reaction of the formation of starch from glucose as shown above.
Acids and enzymes can help the digestion of starch.
Enzymes Enzymes are biological catalyst and were covered in Unit 1: Reaction rates.
Enzymes such as amylase help to break down starch into glucose molecules.
These enzymes function best at body temperature (37°C) and are destroyed at higher temperatures.
Proteins
Proteins form an important class of food made by plants. When animals eat plants, we take in these proteins and use/modify them for our own purposes. Animals (including humans) use proteins as the main material to build tissue, muscle, hair, fingernail, etc. Proteins are also used in the maintenance and regulation of life processes and include enzymes and many hormones, e.g. insulin and haemoglobin.
How are proteins made?
Proteins are made when amino acids join together.
Amino acids are molecules that contain an amine
group (-NH2) at one end of the molecule and a carboxylic acid group at the other (-COOH).
In between these groups is a carbon chain (shown by the blue box) that can vary in its composition. This makes it possible to have a huge variety of different amino acids.
When these molecules join together, they eliminate water molecules and form a large molecule called a protein.
Proteins are formed by condensation polymerisation of amino acids as shown below.
The peptide link The structure of a section of protein is based on the constituent amino acids. Condensation of amino acids produces the peptide (amide) link which is shown on the left. The peptide link is formed by the reaction of an amine group with a carboxyl group.
During digestion, enzymes break apart the large insoluble protein molecules (by hydrolysis) to produce the component amino acid molecules. This is basically the reverse of the reaction above.
The amino acid molecules are small enough to be soluble in the bloodstream and can be transported around the body to the location that they are needed.
The body then recombines these amino acids into the desired order so as to produce a specific protein that the body requires.
It is possible to identify the individual amino acids in each protein as each amino acid in the protein is separated from the next by the peptide link.
The amino acids produced when a protein is hydrolysed can be identified by chromatography. Hydrolysis is the process where the amine link is broken using water molecules. Hydrolysis is the reverse of condensation.
Fats and oils
Fats and oils in the diet supply the body with energy and are a more concentrated source of energy than carbohydrates.
Natural fats and oils can be classified according to their origin:
Animal Vegetable Marine
Beef fat Almond oil Cod liver oil Pork fat Olive oil Shark liver oil Sunflower oil Whale oil Evening primrose oil
Fats and oils can also be termed as saturated and unsaturated. These terms were covered in the 'Reactions of carbon compounds' topic.
Saturated compounds do not contain carbon to carbon double or triple bonds - only single carbon to carbon bonds are present. Unsaturated compounds contain carbon to carbon double and/or triple bonds.
As with alkenes, the test for an unsaturated fat or oil molecule is that it decolourises bromine water rapidly.
Differences between fats and oils
Fats are solid and oils are liquids at room temperature.
Fats tend to contain more saturated molecules (less double bonds) than oils and this causes them to have higher melting points. The lower melting points of oils compared to those of fats is related to the higher unsaturation (more double bonds) of oil molecules.
Oils can be converted into hardened fats by the partial removal of unsaturation by addition of hydrogen. (Addition reactions involving hydrogen were covered in the 'Reactions of carbon compounds' topic). This reaction is called hydrogenation.
The structure of fats and oils
Fats and oils are esters. This means that they are formed when molecules that contain carboxylic acid groups join with molecules containing an alcohol group. (this was covered in the 'Reactions of carbon compounds' topic.)
The acids that make fats and oils are called fatty acids and are straight chain saturated or unsaturated carboxylic acids.
Capric acid is a fatty acid found in goats milk. It is a completely saturated fatty acid
An example of an two unsaturated fatty acids are shown below.
Essential fatty acids (some of which are shown above) are essential for our bodies to function normally and are used in the structure of cell walls, etc. and collectively they are called Vitamin F
In fats and oils, the alcohol groups are provided by the compound propane-1,2,3-triol, which is commonly known as glycerol.
Since glycerol contains three alcohol (-OH) groups, it can combine with three fatty acids to produce a fat or an oil.
The reaction between three fatty acids and glycerol is shown below. The reverse reaction can also take place, when the fat/oil is hydrolysed to form glycerol and three fatty acids.
NEW WORDS AND THEIR MEANINGS
CARBOHYDRATE - a compound containing carbon, hydrogen and oxygen in which the ratio of hydrogen:oxygen is the same as in water (2 hydrogens for each oxygen).
PHOTOSYNTHESIS - a process in plants in which carbon dioxide and water are changed into carbohydrates and oxygen with the help of sunlight and chlorophyll.
RESPIRATION - a process in living things where oxygen is used to break up food and produce water, carbon dioxide and energy.
SUGAR - a molecule made by plants that give us energy. This is a simple carbohydrate.
STARCH - a polymer made from glucose molecules. This is the form that plants store their carbohydrates.
BENEDICT'S TEST - a test that detects sugars (except sucrose). In the test, Benedict's solution turns from blue to brick red if a sugar is present.
IODINE TEST - a test that detects starch. Iodine solution turns from reddish-brown to blue-black in the presence of starch.
PROTEIN - a molecule that is made from many amino acids. Proteins are used for body-building and body repair.
AMINO ACIDS - molecules that contain an amine group and a carboxylic acid group at either end of the molecule. These join together to form proteins.
PEPTIDE LINK - the link that is between amino acids when they form a protein. The link is -CONH-
FAT - solid esters formed when fatty acids join up with glycerol. They are an important class of foodstuffs.
OIL - liquid esters formed when fatty acids join up with glycerol. They are an important class of foodstuffs..
SATURATED - compounds that only contain single bonds between carbon atoms.
UNSATURATED - compounds that contain double (or triple) carbon to carbon bonds.
HYDROGENATION - The process where hydrogen is added across a double carbon to carbon bond in an unsaturated fat/oil to reduce the level of unsaturation (or increase the level of saturation).
FATTY ACIDS - a group of molecules that contain a straight carbon chain with a carboxylic acid group at one end. Fatty acids are used to make fats and oils.
GLYCEROL - a molecule that contains three alcohol groups (-OH) that is used in the formation of fats/oils when it combines with three fatty acids. Unit 3 Acids, Bases and Metals
The pH scale
The pH scale is a continuous range of numbers from below 0 to above 14 which indicate the acidity or alkalinity of solutions.
Acids have a pH of less than 7. (pH<7) Alkalis have a pH of more than 7. (pH>7) Pure water and neutral solutions have a pH equal to 7. (pH=7) Solids and gases do not have a pH value, they need to be dissolved in water before pH can be measured.
Non-metal oxides
Non metal elements can react with air or oxygen to form compounds called oxides.
C + O2 ------> CO2
S + O2 ------> SO2
N2 + 2O2 ------> 2NO2
Non-metal oxides which dissolve in water produce acid solutions, e.g. CO2, NO2, SO2, SO3. Oxides of non-metals which do not dissolve do not affect the pH.
Metal oxides
Oxides of metals or hydroxides of metals which dissolve in water produce alkaline solutions. The data book (page 5) gives information about which ones dissolve or react.
Na2O + H2O ------> 2NaOH
The oxides of Group 1 metals and some of some Group 2 metals produce alkaline solutions with water. Ammonia, which is not an oxide, also dissolves in water to produce an alkali.
Acids in the Laboratory and in the home
Formula pH Uses
Chemical Name Hydrochloric acid HCl 1-3 Common laboratory acid
Nitric acid HNO3 1-3 Common laboratory acid
Sulphuric acid H2SO4 1-3 Common laboratory acid Vinegar N/A 4-6 Common household acid Lemon juice N/A 4-6 Common household acid Car battery acid N/A 1-3 Common household acid
Alkalis in the Laboratory and in the home
Formula pH Uses
Chemical Name Sodium hydroxide NaOH 11-14 Common laboratory alkali Potassium hydroxide KOH 11-14 Common laboratory alkali
Calcium hydroxide Ca(OH)2 11-14 Common laboratory alkali Cleaning fluids N/A 8-14 Common household alkali Toothpaste N/A 8-11 Common household alkali Indigestion tablets N/A 8-11 Common household alkali
Hydrogen and hydroxide ions
Water molecules can break up to form hydrogen ions and hydroxide ions as follows:
+ - H2O (l) H (aq) + OH (aq)
The double headed arrow between the reactants and the products shows a reversible reaction. A small proportion of water molecules break up (forward reaction), but a large proportion of these ions formed join back up together to form the water molecules (reverse reaction). At any one time, there are far more molecules than ions in pure water. For every mole of hydrogen ions and hydroxide ions there are approximately 555,000,000 moles of water molecules.
Water molecules are constantly breaking up and reforming. The speed of the forward reaction equals the speed of the reverse reaction, with both reactions taking place all of the time and the overall concentration of the reactants (water molecules) remains constant as well as the overall concentration of the products (hyrogen and hydroxide ions) remaining constant. This means the reversible reaction is in equilibrium. Note that the concentration of the reactants and the products do not need to be the same for it to be in equilibrium.
In water and neutral solutions the concentration of hydrogen ions, H+(aq), is equal to the concentration of hydroxide ions OH-(aq). Acid solutions contain more hydrogen ions than hydroxide ions. Alkaline solutions contains more hydroxide ions than hydrogen ions. The ions formed when water molecules dissociate (break up), helps to explain why water, although made of molecules, can conduct electricity (due to the ions made).
Dilution of acids and alkalis
When we dilute acids and alkalis, we are effectively adding more water molecules (only a tiny proportion of which dissociate into ions). This means that the concentration of these ions in the acids and alkalis decrease.
Diluting acids decreases the concentration of H+(aq) ions. This means that the pH increases towards 7 Diluting alkalis decreases the concentration of OH-(aq) ions. This means that the pH decreases towards 7
Concentration
Solutions are formed when solutes dissolve in solvents. If the moles of solute and the volume of solvent used is know, the concentration can be calculated.
The concentration of a solution is expressed in mol l-1 and can be calculated as follows:
Concentration = Number of moles Volume
The number of moles of solute, volume and concentration of a solution can be calculated from the other two variables.
Some example questions
1. If 30g of sodium hydroxide is dissolved in 500 ml of water, what will be the concentration of the solution?
If 1 mole of NaOH weighs (23 + 16 +1) = 40g Then number of moles in 30g = 30g / 40g = 0.75 moles From the moles / volume / concentration triangle - Concentration (in mol l-1) = Number of moles / Volume of solution in litres 500 ml = 500 / 1000 = 0.5 litres Concentration = 0.75 / 0.5 = 1.50 mol l-1
2. If the concentration of a solution of hydrochloric acid is 0.5 mol l-1, what mass of acid will be present in 100 ml (0.1 litres) of this acid? Number of moles = Concentration x Volume = 0.5 x 0.1 = 0.05 moles of Hydrochloric acid 1 mole of HCl weighs 1 + 35.5 = 36.5g So 0.05 moles weighs 36.5 x 0.05 = 1.825 g
Strong and weak acids and bases
When acids and alkalis dissolve in water, the compounds are split up (dissociated) by the water molecules to form ions.
Strong acids, such as hydrochloric acid, are completely dissociated in water to form ions: HCl(g) + (aq) H+(aq) + Cl-(aq) Strong acids include hydrochloric, nitric and sulphuric acid Weak acids, such as ethanoic acid, only partially dissociate in water. Only some ions are formed, and these ions are in equilibrium with the starting compound: + - CH3COOH(l) + (aq) H (aq) + CH3COO (aq) Strong bases, such as sodium hydroxide, are completely ionised in water: NaOH(s) + (aq) Na+(aq) + OH-(aq) Solutions of metal hydroxides are strong bases (the metal hydroxide has to be soluble to form a solution) Weak bases, such as ammonia, are only partially ionised in water:
+ - NH3(g) + H2O(l) NH4 (aq) + OH (aq)
Properties of weak and strong acids and bases
Since strong acids and bases form ions more easily than weak acids and bases, their properties are slightly different
pH comparisons
Equimolar solutions of strong acids have lower pH numbers (more 'acid') than weak acids Equimolar solutions of strong bases have higher pH numbers (more 'alkali') than weak alkalis
Conductivity comparisons
Because strong acids and bases form more ions, they are better at conducting electricity than weak acids and bases.
Rate of reaction
Due to the increase in acidity/alkalinity, equimolar solutions of strong acids/bases react quicker than weak acids/bases.
NEW WORDS AND THEIR MEANINGS
PH SCALE - A scale that ranges from below 0 to above 14 that is a measure of acidity/alkalinity of a solution.
REVERSIBLE REACTION - A chemical reaction in which reactants form products and products can also reform the reactants.
EQUILIBRIUM - A reversible reaction when the concentration of reactants and products remains constant (though not necessarily the same as each other).
DISSOCIATE - The break up of compounds by water to form ions.
Alkalis and bases
Bases are substances that can neutralise acids. Alkalis are formed when bases (normally metal oxides, which are called basic oxides) dissolve in water forming hydroxide ions (OH-). Alkalis have a higher concentration of OH- than H+ ions. Soluble bases form solutions (alkalis) which have pH numbers greater than 7. Insoluble bases can still neutralise acids, but do not have any effect on pH indicator. Metal oxides, metal hydroxides and metal carbonates are examples of bases.
Some examples of bases, and alkalis formed are shown in the table below:
Soluble/Insoluble Base Alkali formed (yes/no) in water
sodium oxide soluble yes
copper oxide insoluble no
iron hydroxide insoluble no
potassium oxide soluble yes
calcium carbonate insoluble no
All of the above substances can neutralise acids (acids are substances that dissolve in water to produce the hydrogen ion, H+).
Neutralisation
Neutralisation is the reaction of acids with bases (or alkalis).
Neutralisation moves the pH of an acid up towards 7. Neutralisation moves the pH of an alkali down towards 7.
Reactions of Acids with Neutralisers (examples of neutralisation)
Acid + Alkali ------> Salt + Water Acid + Metal Oxide ------> Salt + Water Acid + Metal Carbonate ------> Salt + Water + Carbon dioxide Acid + Metal ------> Salt + Hydrogen
1. When acids react with alkalis, the hydrogen ions from the acid (H+) reacts with the hydroxide ion from the alkali (OH-) to form water as shown below: + - H (aq) + OH (aq) H2O(l) 2. When acids react with metal oxides, the hydrogen ions from the acid (H+) reacts with the oxide ion from the metal oxide (O2-) to form water as shown below: + 2- 2H (aq) + O (aq) H2O(l) 3. When acids react with metal carbonates, the hydrogen ions from the acid (H+) reacts with 2- the carbonate ion from the metal carbonate (CO3 ) to form water and carbon dioxide as shown below:
+ 2- 2H (aq) + CO3 (aq) H2O(l) + CO2(g)
Everyday neutralisation reactions
Acids are often neutralised in everyday chemical reactions:
The use of indigestion (or ant-acid) tablets to neutralise excess stomach acid. The use of lime to reduce soil acidity. The use of lime to reduce acidity in lochs.
Acids and metals
Some metals react with acids to give off hydrogen gas. The hydrogen gas can be collected and tested with a lit splint. Hydrogen burns with a 'pop'.
When acids react with metals, the hydrogen ions from the acid (H+) form hydrogen molecules as shown below:
+ 2H (aq) H2(g)
Metals such as copper, silver and gold do not react with dilute acids.
Acid rain Sulphur dioxide, produced by the burning of fossil fuels, and nitrogen dioxide, produced by the sparking of air in car engines, dissolve in water in the atmosphere to produce acid rain (this was covered in the Fuels topic).
Acid rain has damaging effects on buildings made from carbonate rock, structures made of iron and steel, soils and plant and animal life.
Volumetric titrations
The concentration of acids/alkalis can be calculated from the results of a volumetric titration.
After carrying out a titration it is important to be able to write a balanced chemical equation for the reaction and to be able to use the concentration formula triangle. From this, you can work out the following formulae:
Concentration = number of moles / volume Number of moles = concentration * volume Volume = number of moles / concentration
Concentration is measured in moles per litre, which is shown as mol l-1, or mol/l Volume in these formulae is always written in litres, which is shown as l
A titration was carried out between sulphuric acid solution and sodium hydroxide solution. The concentration of the acid was 0.1150 mol l-1 and 28.60 ml of it was used to neutralise 20.00 ml of the sodium hydroxide solution. The reaction formed sodium sulphate (a salt) and water
What was the concentration of the sodium hydroxide solution?
1) Write a balanced H SO + 2NaOH ---> Na SO + 2H O chemical 2 4 2 4 2 equation 2) Identify the moles of 1 mole of acid reacts with 2 moles of alkali reactants involved 3) Calculate the Number of moles = concentration * volume (in litres) moles of acid Number of moles = 0.1150 mol l-1 * 0.02860 l actually used Number of moles = 0.003289 moles 4) Calculate the number of moles of sodium 1 mole of acid reacts with 2 moles of alkali hydroxide used 0.003289 moles of acid reacts with (2*0.003289) moles of alkali from the 0.003289 moles of acid reacts with 0.006578 moles of alkali balanced chemical equation 5) Calculate the Concentration = number of moles / volume (in litres) concentration Concentration = 0.006578 / 0.02000 l of the sodium Concentration = 0.3289 mol l-1 hydroxide The concentration of the sodium hydroxide solution is 0.3289 mol l-1
Note : When carrying out these calculations, never round off values halfway through the calculation - you should only round your answer at the final stage. Also, never give too many decimal places in your answer. In the above example, the concentration of the acid was given to 4 decimal places, so it is not possible to calculate the concentration of the alkali to any more precision than 4 decimal places.
Naming salts
A salt is a compound in which the hydrogen ions of an acid have been replaced by metal ions (or ammonium ions). Salts are formed in the reaction of acids with bases or metals.
1. Hydrochloric acid (HCl) always makes CHLORIDE salts
2. Nitric acid (HNO3) always makes NITRATE salts
3. Sulphuric acid (H2SO4) always makes SULPHATE salts
NB. Learn the formulae of these acids. NB. The acid forms the 'Surname' of the salt. NB. The neutraliser forms the 'Forename' of the salt
e.g.
1. If hydrochloric acid is neutralised by potassium hydroxide, the salt potassium chloride will be formed (+ water) 2. If sulphuric acid is neutralised by sodium carbonate, the salt sodium sulphate will be formed (+ water + carbon dioxide) 3. If nitric acid is neutralised by sodium carbonate, the salt sodium nitrate will be formed (+ water + carbon dioxide) 4. If hydrochloric acid is neutralised by zinc metal, the salt zinc chloride will be formed (+ hydrogen)
Some nitrogen salts, including ammonium nitrate, ammonium sulphate and potassium nitrate are made by neutralisation reactions for use as fertilisers; these salts are soluble in water.
In the preparation of a soluble salt, it is often easier to use an insoluble metal carbonate or metal oxide as the base, so that the excess base can be filtered off before evaporating the reaction mixture to obtain the salt.
Precipitation
Precipitation is the reaction of two solutions to form an insoluble product called a precipitate. Insoluble salts can be formed by precipitation.
To make insoluble lead (II) iodide, mix together two separate solutions of soluble salts one containing lead (II) ions and the other containing iodide ions e.g. lead (II) nitrate solution and sodium iodide solution. Pb(NO3)2(aq) + 2NaI(aq) ------> PbI2(s) + 2NaNO3(aq)
Lead iodide is formed as a yellow precipitate which can be removed easily by filtering, followed by washing the precipitate with distilled water and then drying it. The method is quick and doesn't need careful measurement as excess reactants are soluble and are left in solution (and are not removed by the filtration process).
The data book indicates the solubility of many substances. e.g. Will a precipitate be formed if sodium carbonate solution is added to copper (II) sulphate solution?
As these two chemicals are both soluble salts, there will only be a precipitate formed if they can react to give an insoluble substance.
Possible products are sodium sulphate and copper (II) carbonate. The data book indicates that copper (II) carbonate is insoluble and so copper (II) carbonate will be formed as a precipitate and sodium sulphate (which is soluble) will be left in the solution.
In general, to make the insoluble salt XY, mix solutions of X nitrate and sodium Y.
Ionic equations
Spectator ions are ions which are free to move (aqueous or molten) at the beginning and end of a chemical reaction, and can therefore be omitted.
Some examples follow:
Acid and alkali
Acid and metal oxide
Acid and metal carbonate Acid and metal
NEW WORDS AND THEIR MEANINGS
BASE - A substance that can neutralise an acid, such as a metal oxide, metal hydroxide, metal carbonate, or a metal.
BASIC OXIDE - A metal oxide.
ALKALI - Formed when a base dissolves in water.
NEUTRALISATION - The reaction between an acid and a base.
VOLUMETRIC TITRATION - A chemical technique carried out using a burette that is used to meause a volume of chemical used in a chemical reaction (volumetric titration is often simplified to titration).
PRECIPITATION - A chemical reaction that produces a solid in a liquid.
CONCENTRATION - The number of moles of a solute dissolved in 1 litre of solvent.
SALT - Formed in a neutralisation reaction. This is an ionic compound containing the metal part (or ammonium ion) of the base and the non-hydrogen part of the acid, e.g. sodium chloride is a salt formed from sodium oxide and hydrochloric acid.
Cells
Chemical reactions can produce electricity.
A cell (often referred to as a battery) contains chemicals which react to make electricity.
The energy change is:
Chemical Energy ------> Electrical Energy
In dry cells, the chemicals are used up and the cells then have to be replaced.
In a simple dry cell, the chemicals are shown in the diagram below. The paste containing ammonium chloride is the electrolyte needed to complete the circuit.
Rechargeable cells.
In rechargeable cells the chemicals are not used up and can be regenerated by recharging the cell.
Energy Changes in Cells
Using the cell (Discharging):
Chemical Energy ------> Electrical Energy
Charging the cell:
Electrical Energy ------> Chemical Energy
Lead-sulphuric acid battery (Car battery)
This is charged using electricity and a brown coating appears on the positive lead plate. When the charger is removed and replaced by a bulb, the bulb lights.
Gradually the bulb dims as the chemical energy in the brown coating is used up and the cell is discharged. The cell now has to be recharged.
The electrochemical series Electricity is produced when two different metals are dipped in an electrolyte and connected together with a wire.
There is a flow of electrons in the wire from one metal to the other, and ions move through the electrolyte.
The data book (Page 7) gives a list of metals in a table called the electrochemical series.
When two different metals are joined together as shown in the above cell, electrons always flow through the wire from the metal higher in the electrochemical series to the metal lower in the series e.g. from Zinc to Copper.
If a voltmeter is used in the wire between the two metals, a different voltage is obtained when different metals are used.
The voltage values can be used to put the metals into the order shown in the electrochemical series e.g. a larger voltage is obtained when magnesium is connected to copper than when zinc is connected to copper, and magnesium is placed above zinc in the electrochemical series.
The reactions of metals with acids (which contain the hydrogen ion) can be used to place hydrogen in the reactivity series.
Electricity from different metals in solutions of their own ions
Electricity can be produced in a cell by connecting two different metals in solutions of their metal ions.
The ammeter will show a flow of electrons through the wire between the two metals always from the metal higher in the electrochemical series to the one lower in the series.
If the ion bridge (or salt bridge) is removed, the current stops flowing through the ammeter.
This happens because the ion bridge is needed to complete the circuit.
By separating the reaction in this way, the cell is divided into two half cells.
In the cell above the two reactions are:
Zn ------> Zn2+ + 2e-
Cu2+ + 2e------> Cu Note that these two equations are in the data book and the equation for the metal higher in the list has been reversed.
They explain how electrons made at zinc travel through the wire to copper where they join onto copper(II) ions to make copper metal.
This is similar to the displacement reaction (see notes later in this page) that occurs when zinc metal is added to a solution which contains copper(II) ions e.g. copper(II) sulphate.
The ion bridge completes the circuit by allowing ions to move through it.
Electricity from cells where at least one of the half cells does not involve a metal
The electrochemical series in the data book has some reactions that involve non-metals e.g.
- - I2 + 2e ------> 2I
A cell can be set up by using a carbon rod dipping into a solution of iodine dissolved in potassium iodide solution (which contains iodide ions) as one half cell and connecting this to another half cell as shown below:
The diagram shows that electrons flow from zinc metal to the carbon rod and the reactions are shown below
Zn ------> Zn2+ + 2e-
- - I2 + 2e ------> 2I
These equations are in the data book.
Displacement reactions
When grey magnesium metal is added to blue copper(II)sulphate solution, brown copper metal is made and the solution becomes colourless.
In this reaction magnesium has pushed copper out of copper(II) sulphate solution as shown in the following equation.
Mg(s) + CuSO4(aq) ------> MgSO4(aq) + Cu(s) blue colourless brown A displacement reaction will happen when a metal higher in the electrochemical series is added to a solution containing a metal lower in the electrochemical series The metal which is lower in the series is displaced or pushed out of its compound which is in solution.
When copper metal is added to colourless silver(I) nitrate solution, the colour changes will be the loss of the brown copper, the appearance of silver metal and the solution turning blue as the copper dissolves.
Cu + 2AgNO3 ------> Cu(NO3)2 + 2Ag brown colourless blue silver
Copper has displaced silver from a compound containing silver ions.
+ - 2+ - Cu + 2Ag NO3 ------> Cu (NO3 )2 + 2Ag
When the nitrate spectator ions are removed, the reaction is between copper atoms and silver(I) ions.
Cu + 2Ag+ ------> Cu2+ + 2Ag
The reactions of metals with acids (which contain the hydrogen ion), used to place hydrogen in the reactivity series is a further example of a displacement reaction.
In this reaction, hydrogen is displaced from an acid by a metal. As with other displacements, only metals above hydrogen can cause this to happen. e.g. magnesium will react but copper will not. As lead will displace hydrogen from an acid, hydrogen is placed between lead and copper in the electrochemical series.
Oxidation
An oxidation reaction is when a reactant loses electrons in a chemical reaction.
An example of this is when magnesium metal is used to displace zinc from zinc sulphate solution. The magnesium loses electrons during the displacement reaction to form magnesium ions. This means the magnesium has been oxidised.
Mg(s) ------> Mg2+ + 2e-(aq)
Reduction
An reduction reaction is when a reactant gains electrons in a chemical reaction.
An example of this is when copper ions are displaced from solution by a more reactive metal. The copper ions gain electrons during the displacement reaction to form copper metal. This means the copper ions have been reduced.
Cu2+ + 2e-(aq) ------> Cu(s)
Redox Reactions
When both oxidation and reduction occur together, the complete reaction is called a REDOX reaction.
If magnesium metal was added to copper sulphate solution, the magnesium metal would be oxidised, while the copper ions were being reduced. This is an example of a redox reaction.
Both of the equations (known as ion-electron equations) can be written and combined, by eliminating the electrons involved, to produce a redox equation:
Example 1: Magnesium metal displacing copper metal from copper(II) sulphate solution
Example 2: aluminium metal displacing silver metal from silver(I) nitrate solution Example 3: zinc metal displacing iron metal from iron(III) sulphate solution
The use of metals
Metals need to be recycled because they will not last forever.
How long a metal can last can be found from the previous bar graphs. Metals are not finite.
Large quantities of metals are thrown away and the need for recycling is shown below:
Reactions of metals
a) With water
Metal + Water ------> Metal hydroxide + Hydrogen
Potassium reacts vigorously, sodium very quickly, calcium quickly and magnesium slowly.
Potassium + Water ------> Potassium hydroxide + Hydrogen
K + H2O ------> KOH + H2
Sodium + Water ------> Sodium hydroxide + Hydrogen
Na + H2O ------> NaOH + H2
Calcium + Water ------> Calcium hydroxide + Hydrogen
Ca + H2O ------> Ca(OH)2 + H2
Magnesium + Water ------> Magnesium hydroxide + Hydrogen
Mg + H2O ------> Mg(OH)2 + H2
The order of metals reacting with water (most reactive first) is :
Potassium, sodium, calcium and magnesium.
b) Metal reacting with Acid Metal + Acid ------> Salt + Hydrogen
Magnesium + Hydrochloric acid ------> Magnesium chloride + Hydrogen
Mg + HCl ------> MgCl2 + H2
The order of reaction can be obtained by observation of the rate at which gas is given off.
All metals above hydrogen in the electrochemical series react with acids to displace hydrogen gas. The order of metals reacting with acid (most reactive first) is
Magnesium, aluminium, zinc, iron, tin and lead.
Copper, mercury, silver and gold do not react, while potassium, sodium and calcium are too reactive to add to acid.
c) Metals reacting with oxygen
Oxygen can be made by heating potassium permanganate in a test tube and allowing the gas to pass through the preheated metal as shown.
A glow spreads through the metal (exothermic reaction), and the speed is related to the relative activity of the metal.
Metal + Oxygen ------> Metal oxide
Magnesium + Oxygen ------> Magnesium oxide
Mg + O2 ------> MgO
The order of metals reacting with oxygen (most reactive first) is
Magnesium, aluminium, zinc, iron, tin, lead, copper and mercury.
Silver and gold do not react, while potassium, sodium and calcium are too reactive to react with oxygen in this way.
These reactions give an indication of the reactivity of the metal and are summarised below:
Metal ores
Ores are naturally-occuring compounds of metals from which metals can be extracted. The three main types of ore are metal carbonates, metal oxide and metal sulphides.
Common name Chemical name Metal present Haematite Iron oxide Iron Bauxite Aluminium oxide Aluminium Galena Lead sulphide Lead Cinnabar Mercury sulphide Mercury Malachite Copper(II) carbonate Copper
Elements on earth
Metals such as gold and silver occur uncombined on earth because they are unreactive and because of this these elements were among the first to be discovered.
Other metals, such as those in the table above are found in compounds and have to be extracted.
Extraction of metals from ores The demand for metals is high and methods are now available to extract all metals from their ores. Methods using carbon (coke) are cheaper and have been used longer than methods which use electricity.
Methods of extraction
a) Heating metal oxides Silver oxide ------> Silver + Oxygen
Ag2O ------> Ag + O2
Few metals can be obtained in this way
b) Heating metal oxides with carbon
The main reaction is : Metal oxide + Carbon ------> Metal + Carbon dioxide
Iron oxide + Carbon ------> Iron + Carbon dioxide
Fe2O3 + C ------> Fe + CO2
This method is used to extract metals below aluminium in the reactivity series.
c) Heating with carbon monoxide
Iron is extracted from its ore in the blast furnace by heating with carbon (coke) in the presence of air.
At the bottom of the furnace the reaction makes carbon dioxide (Zone 1)
C + O2 ------> CO2
Higher up, the carbon dioxide reacts with carbon to make carbon monoxide (Zone 2)
CO2 + C ------> CO
Further up the carbon monoxide reacts with iron oxide to make iron and carbon dioxide. (Zone 3)
The formation of a metal from a compound is known as reduction. Fe2O3 + CO ------> Fe + CO2
d) Using electricity
Electricity can be used to split ionic compounds into their elements in a process called electrolysis. The method is used to extract reactive metals above zinc in the reactivity series.
A large electric current is passed through the molten compound, and metal appears at the negative electrode. At the negative electrode, reduction is taking place (oxidation is taking place at the positive electrode).
Aluminium oxide ------> Aluminium + Oxygen
The method used to extract a metal depends on the reactivity of the metal.
The more reactive the metal, the more difficult it is to extract. The less reactive the metal, the easier it is to extract.
Electricity is used to extract the most reactive metals such as potassium, sodium, calcium, magnesium and aluminium.
Corrosion
Corrosion is a chemical reaction which involves the surface of a metal changing from an element to a compound. This natural change of metals into compounds is very costly.
Speed of Corrosion
Most metals corrode, but the speed at which they corrode is related to the chemical activity series. Metals high in the reactivity series (such as potassium) corrode very quickly while those lower in the series corrode much more slowly (such as silver and gold)
Rusting
Rusting is the special name given to the corrosion of iron As iron, in the form of steel, is the most commonly used metal in the world, the corrosion of iron is important.
The Cause of Rusting
The experiment to the right shows that oxygen and water are both needed for rusting to occur.
Further proof that oxygen is needed is seen in the experiment on the left.
As about 80% of the air in the cylinder is left after rusting - it means that oxygen is used up during rusting and water rises to take its place.
Detecting Rusting
The typical brown colour of rust is the end result of rusting. The first stage of the rusting process can be detected by ferroxyl indicator. As a blue colour is only made with iron(II) chloride solution, and with a rusting nail, it proves that iron(II) ions (Fe2+) are made during rusting. Fe2+ are produced when iron atoms lose 2 electrons.
Corrosion is an example of oxidation because it involves a loss of electrons.
Fe ------> Fe2+ + 2e-
The rusting process continues when iron(II) ions lose another electron to form iron(III) ions.
Fe2+ ------> Fe3+ + e-
The iron(III) ions can be shown using a colourless solution of ammonium thiocyanate. The solution will turn blood-red.
The electrons 'lost' when iron is oxidised during the rusting process are accepted by water and oxygen (the requirements for rusting) and are shown in the following equation (this equation is given in the data booklet).
- 2H2O + O2 + 4e ------> 4OH
These hydroxide ions can also be detected by ferroxy indicator, which turns pink to indicate their presence.
As iron atoms lose electrons in rusting and oxygen/water molecules gain these electrons, rusting is described as a Redox reaction.
Increasing the rate of corrosion
Corrosion requires an electrolyte such as dissolved salt or acid rain. Acid can speed up corrosion in two ways:
(a) by acting as an electrolyte
(b) by reacting with the metal.
Electrolytes increase the speed of rusting, and cars rust faster in winter when salt is spread on the roads.
The electrolyte helps to carry ions away from the rusting iron and this speeds up the oxidation (corrosion).
Using a battery to prevent the rusting of iron The battery causes the nail connected to the positive terminal to rust rapidly, but the nail connected to the negative terminal does not rust.
Iron has to lose electrons in order to rust. The negative terminal of the battery is pushing electrons onto this nail and this prevents this nail from losing any electrons. This nail cannot rust. Ferroxyl indicator turns pink because hydroxide ions are made here. Electrons flowing to the nail stop rusting. The positive terminal of the battery is removing electrons from the nail connected to it. This nail rusts rapidly, changing into iron(II) ions which turn ferroxyl indicator blue. Electrons flowing from the nail increase rusting.
Connecting different metals to iron
Metals that push electrons onto iron stop rusting, but metals that let electrons flow from iron increase the speed of rusting.
Metals that push electrons onto iron are higher than iron in the reactivity series (such as magnesium) Metals that let electrons flow from iron are lower than iron in the reactivity series (such as copper)
Preventing Corrosion
A. Physical Protection
This is where a metal is given a coating to stop it coming in contact with air and water and thus prevents corrosion.
Methods available for physical protection
painting e.g. the Forth Rail Bridge. greasing or oiling - protects moving parts of machinery. coating with plastic - dish drainers have a metal core and a plastic coating. coating with other metals such as tin, zinc, silver, gold.
Tin-plating - metals can be coated with other metals which are less likely to corrode. Food cans are steel cans dipped into molten tin giving a layer of tin.
Electroplating - e.g. chromium-plating of car bumpers and the silver-plating of cutlery are done using this process to give an attractive appearance which provides protection against corrosion.
Galvanising - galvanised iron in made by dipping iron into molten zinc which coats the iron with zinc. It is used to protect dustbins, car exhausts and special nails.
B. Chemical Protection Tin-plating works well provided the layer of tin remains unbroken. If the tin layer to scratched, the iron corrodes quickly because electrons travel to tin from iron.
Zinc-plating works well if the layer of zinc remains unbroken and also when scratched because then the zinc corrodes quickly and electrons are pushed onto the iron. This is an example of sacrificial protection - where a more reactive metal is allowed to corrode in order to protect a less reactive metal.
NEW WORDS AND THEIR MEANINGS
BATTERY - a device containing chemicals that react to produce electricity.
CELL - the correct term for devices called batteries.
ELECTROLYTE - a substance that contains ions which can move (either molten ionic compounds or ionic compounds in solution or a watery paste as in a dry cell).
RECHARGEABLE CELL - a cell in which electricity can regenerate chemicals to allow the cell to produce electricity many times.
ELECTROCHEMICAL SERIES - a list of reactions in the data book (page 7) which can be used to determine which reaction of a pair is better at pushing electrons onto the other reaction.
VOLTAGE - a measure of the push of electrons between two reactions.
ION BRIDGE - a link containing ions which completes a circuit by allowing ions to travel through it.
DISPLACEMENT REACTION - where one metal higher in the electrochemical series displaces (pushes out) another metal from a solution of the other metal.
OXIDATION - the loss of electrons by a reactant in any reaction.
REDUCTION - the gain of electrons by a reactant in any reaction.
REDOX REACTION - a reaction in which reduction and oxidation occur together.
ION-ELECTRON EQUATION - an equation that shows ions and electrons involved in a reduction, oxidation or redox reaction.
OILRIG - This will help you remember oxidation and reduction. Oxidation Is Loss, Reduction Is Gain (of electrons).
ORE - a naturally-occuring compound of metals.
ELECTROLYSIS - splitting a substance into its elements using electricity.
CORROSION - the changing of the surface of a metal from an element into a compound.
RUSTING - the special name for the corrosion of iron.
FERROXYL INDICATOR - turns blue in the presence of iron(II) ions and turns pink in the presence of hydroxide ions. PHYSICAL PROTECTION - stopping corrosion by keeping out air and/or water
CHEMICAL PROTECTION - using more reactive metals to push electrons onto iron
GALVANISING - coating iron with a layer of zinc
TIN PLATING - coating iron with a layer of tin
ELECTROPLATING - coating a metal with a layer of another metal using electricity
SACRIFICIAL PROTECTION - where a more reactive metal sacrifices itself to protect the less reactive metal