Solid State Structure-The Cubic Lattice Systems
Special Items: Twenty-seven 1½ inch and thirteen 3 inch Styrofoam balls and double- pointed toothpicks. These are available in from the lab stockroom.
Crystalline solids are characterized by distinctive geometric shapes, which are thought to arise from definite orderly patterns of the constituent atoms. The order of arrangement can be represented by the unit cell. The unit cell is defined as the smallest portion of the space lattice which, moved a distance equal to its own dimensions in various directions, generates the whole space lattice. In this investigation, you will first construct models of the three cubic unit cells representing a simple atomic solid, and assuming that atoms can be represented as rigid spheres, calculate the fraction of the volume that is empty or filled space. Then you will construct a model of the unit cells of sodium chloride and show its relationship to its simplest formula. In addition to the model constructions and calculations, answers to follow-up questions will be required.
PROCEDURE
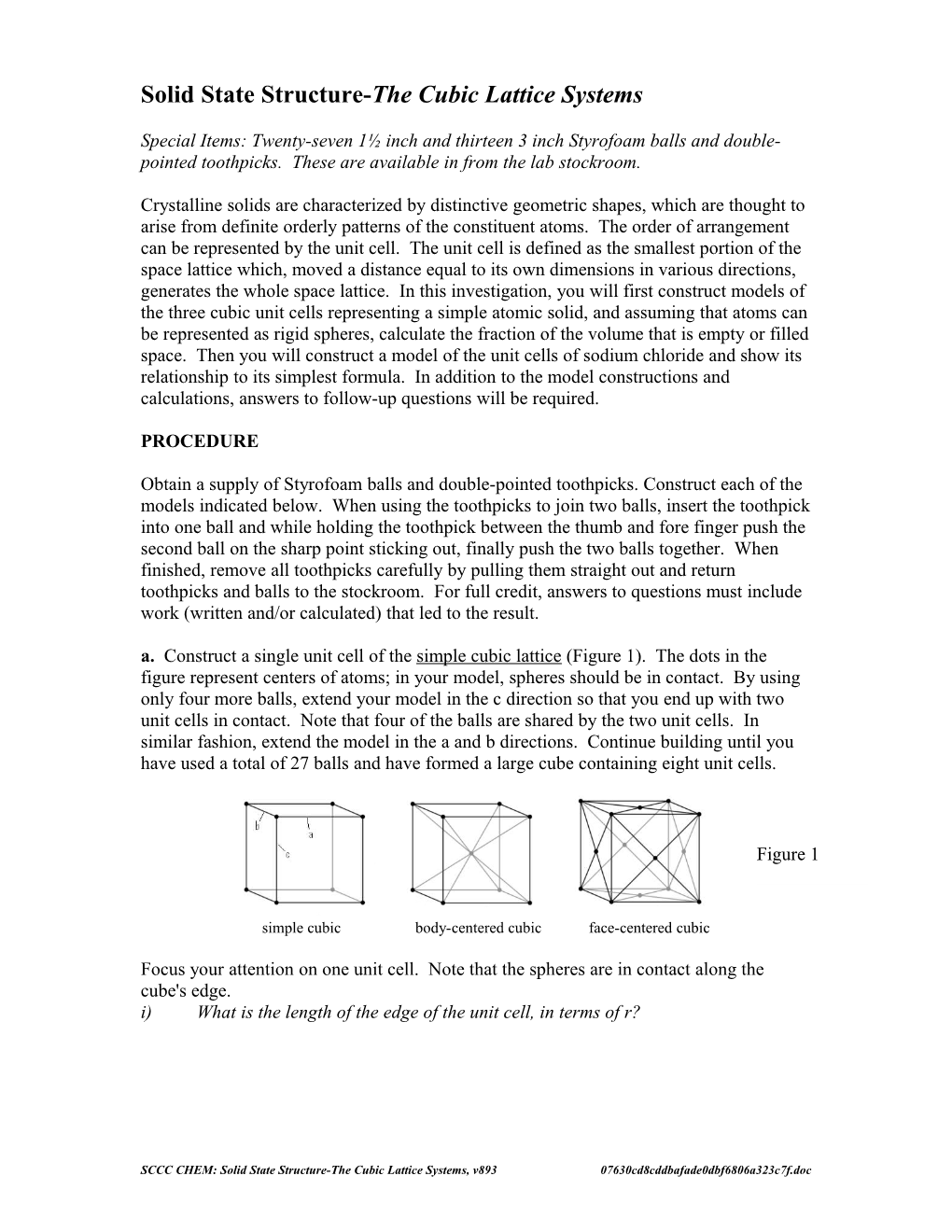
Obtain a supply of Styrofoam balls and double-pointed toothpicks. Construct each of the models indicated below. When using the toothpicks to join two balls, insert the toothpick into one ball and while holding the toothpick between the thumb and fore finger push the second ball on the sharp point sticking out, finally push the two balls together. When finished, remove all toothpicks carefully by pulling them straight out and return toothpicks and balls to the stockroom. For full credit, answers to questions must include work (written and/or calculated) that led to the result. a. Construct a single unit cell of the simple cubic lattice (Figure 1). The dots in the figure represent centers of atoms; in your model, spheres should be in contact. By using only four more balls, extend your model in the c direction so that you end up with two unit cells in contact. Note that four of the balls are shared by the two unit cells. In similar fashion, extend the model in the a and b directions. Continue building until you have used a total of 27 balls and have formed a large cube containing eight unit cells.
Figure 1
simple cubic body-centered cubic face-centered cubic
Focus your attention on one unit cell. Note that the spheres are in contact along the cube's edge. i) What is the length of the edge of the unit cell, in terms of r?
SCCC CHEM: Solid State Structure-The Cubic Lattice Systems, v893 07630cd8cddbafade0dbf6806a323c7f.doc Now focus your attention on the sphere hidden in the center of the 27-ball model. ii) What fraction of this sphere lies in each unit cell?
In one unit cell, determine how many spheres there are (by considering the fractions of spheres at each corner-the corners of the unit cell are at the center of spheres.) iii) Determine the # of spheres in one unit cell.
The coordination number for a unit cell is the number of spheres which contact any one sphere. iv) What is the coordination number for this unit cell? v) Calculate the fraction of the unit cell which is occupied, by dividing the volume 4 3 of spheres (Vsphere 3 r ) within one unit cell by the total volume of the cube. What is the percent of empty space in the unit cell? Show all your work.
b. Construct a single unit cell of the face centered cubic (fcc) lattice, using fourteen atoms and the figure provided for comparison. The spheres are in contact along the cube's face diagonals. Turn the cell 45 degrees and look for the "abcabc" layering of hexagonal closest packing. i) What is the length of one face diagonal, in terms of r?
ii) Determine the # of spheres in one unit cell? iii) What is the coordination number?
Holes within a crystal structure can be classified based on shape (trigonal, tetrahedral, or octahedral). iv) What type of hole is at the very center of the unit cell? v) Determine the # of tetrahedral holes in one unit cell. vi) Calculate the percent of the unit cell which is filled space. To calculate the volume of the cube, you'll have to determine the edge length in terms of r. Set up a triangle with the face diagonal and use the Pythagorean Theorem.
c. Construct a single unit cell of the body centered cubic (bcc) lattice. If you use five spheres, you will have a structure that extends beyond the unit cell somewhat -- the best SCCC CHEM: Solid State Structure-The Cubic Lattice Systems, v893 07630cd8cddbafade0dbf6806a323c7f.doc we can do without cutting up the spheres! The spheres should be in contact along the cube's internal diagonal this time. i) What is the length of one internal diagonal, in terms of r?
ii) Determine the # of spheres in one unit cell? iii) What is the coordination number? iv) Calculate the percent of the bcc unit cell which is empty space, showing all work. The calculation of the edge length, in terms of r, requires quite a bit of trigonometry.
d. Construct a single unit cell of the NaCl lattice, using the small and large spheres. Which size should be used for anions, and which for cations? Start with a cation sphere and attach 4 chlorides at 90 degree angles, in a flat square structure. Put 4 sodium cations between each chloride. This is the middle, horizontal layer of your unit cell. Build up one layer by attaching a chloride on top of each sodium and a sodium on top of each chloride. Do the same to make one bottom layer. Altogether, you will use 13 sodium and 14 chloride spheres. Notice that this overall structure could be described as being fcc for the anions, with the cations filling all the octahedral holes. i) Determine the # of sodium cations per unit cell. ii) Determine the # of chloride per unit cell.
*** For coordination number in ionic compounds, only count contacts with oppositely charged ions *** iii) Determine the coordination number for sodium. iv) Determine the coordination number for chloride. v) Determine the ratio of sodium to chloride.
e. Construct the unit cell of Ag2O, or refer to figure 2.
SCCC CHEM: Solid State Structure-The Cubic Lattice Systems, v893 07630cd8cddbafade0dbf6806a323c7f.doc Ag2O Figure 2 Ag+ 115 pm
O2- 140 pm i) Which ion makes up the fcc arrangement? ii) What type of hole is occupied by the other ion? iii) What percent of the total possible holes of this type are occupied? vi) Determine the number of silver ions per unit cell. v) Determine the number of oxides per unit cell. vi) What is the coordination number for silver? vii) What is the coordination number for oxide? viii) What is the ratio of silver to oxygen in the unit cell? f. Construct the unit cell of Cu3Au, or refer to figure 3.
Cu3Au Figure 3 Cu 128 pm
Au 144 pm
i) What is the closest packed arrangement of all atoms, hcp, ccp (fcc), bcc, or sc? ii) Determine the number of copper atoms per unit cell. iii) Determine the number of gold atoms per unit cell. iv) What is the ratio of copper to gold in the unit cell? v) What is the coordination number of copper (consider only contacts to gold)? vi) What is the coordination number of gold (consider only contacts to copper)? g. For the unit cells constructed in a, b, and c, assume an atomic mass of 50.00 amu (typical for a period 4 d-block element) and an atomic radius of 1.50 Angstroms.
SCCC CHEM: Solid State Structure-The Cubic Lattice Systems, v893 07630cd8cddbafade0dbf6806a323c7f.doc i) Calculate the density of the solid in each arrangement, in g/cm3
a.
b.
c. ii. Which of the above is the densest?
h. CsCl, though its formula is similar to sodium chloride, takes a different unit cell. The chloride anions are simple cubic form, with a cesium cation at the very center. There is still a 1: 1 ratio of cations to anions in each unit cell. What is different about cesium chloride (compared to NaCl) which might cause it to prefer this arrangement? Quantify this difference.
SCCC CHEM: Solid State Structure-The Cubic Lattice Systems, v893 07630cd8cddbafade0dbf6806a323c7f.doc