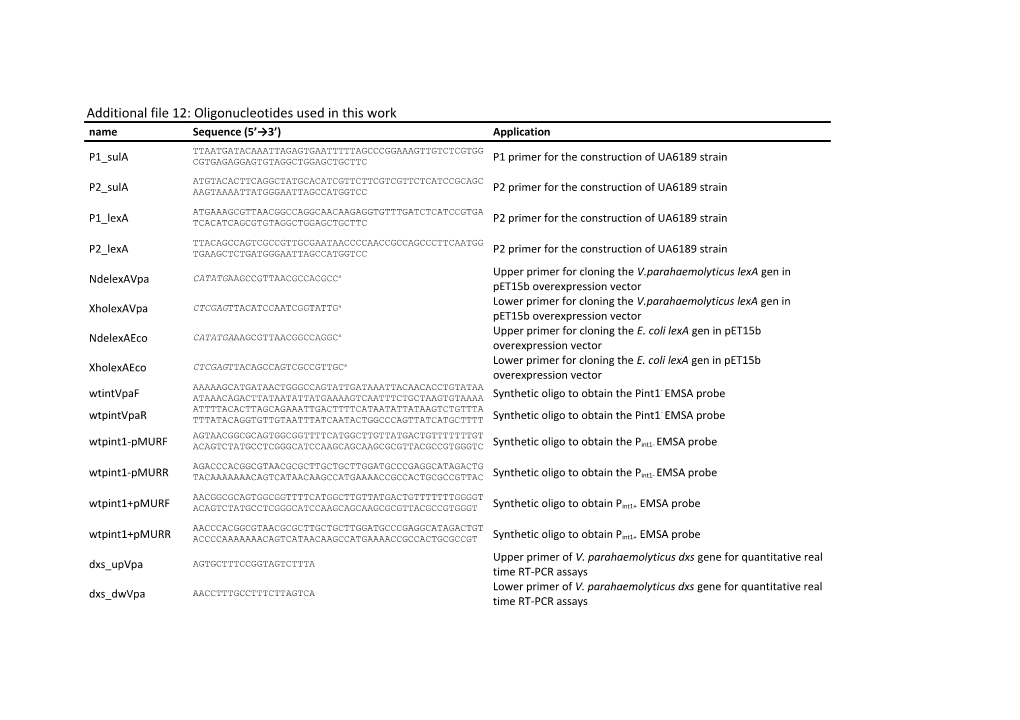
Additional file 12: Oligonucleotides used in this work name Sequence (5’→3’) Application TTAATGATACAAATTAGAGTGAATTTTTAGCCCGGAAAGTTGTCTCGTGG P1_sulA CGTGAGAGGAGTGTAGGCTGGAGCTGCTTC P1 primer for the construction of UA6189 strain ATGTACACTTCAGGCTATGCACATCGTTCTTCGTCGTTCTCATCCGCAGC P2_sulA AAGTAAAATTATGGGAATTAGCCATGGTCC P2 primer for the construction of UA6189 strain
ATGAAAGCGTTAACGGCCAGGCAACAAGAGGTGTTTGATCTCATCCGTGA P1_lexA TCACATCAGCGTGTAGGCTGGAGCTGCTTC P2 primer for the construction of UA6189 strain
TTACAGCCAGTCGCCGTTGCGAATAACCCCAACCGCCAGCCCTTCAATGG P2_lexA TGAAGCTCTGATGGGAATTAGCCATGGTCC P2 primer for the construction of UA6189 strain Upper primer for cloning the V.parahaemolyticus lexA gen in NdelexAVpa CATATGAAGCCGTTAACGCCACGCCa pET15b overexpression vector Lower primer for cloning the V.parahaemolyticus lexA gen in XholexAVpa CTCGAGTTACATCCAATCGGTATTGa pET15b overexpression vector Upper primer for cloning the E. coli lexA gen in pET15b NdelexAEco CATATGAAAGCGTTAACGGCCAGGCa overexpression vector Lower primer for cloning the E. coli lexA gen in pET15b XholexAEco CTCGAGTTACAGCCAGTCGCCGTTGCa overexpression vector AAAAAGCATGATAACTGGGCCAGTATTGATAAATTACAACACCTGTATAA - wtintVpaF ATAAACAGACTTATAATATTATGAAAAGTCAATTTCTGCTAAGTGTAAAA Synthetic oligo to obtain the Pint1 EMSA probe ATTTTACACTTAGCAGAAATTGACTTTTCATAATATTATAAGTCTGTTTA - wtpintVpaR TTTATACAGGTGTTGTAATTTATCAATACTGGCCCAGTTATCATGCTTTT Synthetic oligo to obtain the Pint1 EMSA probe
AGTAACGGCGCAGTGGCGGTTTTCATGGCTTGTTATGACTGTTTTTTTGT wtpint1-pMURF ACAGTCTATGCCTCGGGCATCCAAGCAGCAAGCGCGTTACGCCGTGGGTC Synthetic oligo to obtain the Pint1- EMSA probe
AGACCCACGGCGTAACGCGCTTGCTGCTTGGATGCCCGAGGCATAGACTG wtpint1-pMURR TACAAAAAAACAGTCATAACAAGCCATGAAAACCGCCACTGCGCCGTTAC Synthetic oligo to obtain the Pint1- EMSA probe
AACGGCGCAGTGGCGGTTTTCATGGCTTGTTATGACTGTTTTTTTGGGGT wtpint1+pMURF ACAGTCTATGCCTCGGGCATCCAAGCAGCAAGCGCGTTACGCCGTGGGT Synthetic oligo to obtain Pint1+ EMSA probe
AACCCACGGCGTAACGCGCTTGCTGCTTGGATGCCCGAGGCATAGACTGT wtpint1+pMURR ACCCCAAAAAAACAGTCATAACAAGCCATGAAAACCGCCACTGCGCCGT Synthetic oligo to obtain Pint1+ EMSA probe Upper primer of V. parahaemolyticus dxs gene for quantitative real dxs_upVpa AGTGCTTTCCGGTAGTCTTTA time RT-PCR assays Lower primer of V. parahaemolyticus dxs gene for quantitative real dxs_dwVpa AACCTTTGCCTTTCTTAGTCA time RT-PCR assays Upper primer of E. coli dxs gene for quantitative real time RT-PCR dxs_upEco GACGAACTGCGCCGCTATT assays Lower primer of E. coli dxs gene for quantitative real time RT-PCR dxs_dwEco CCGGCACTGATGGAGGTTGAT assays Upper primer of pMUR050 int gene for quantitative real time RT- EcoInt_up AAACCGAGGATGCGAACCACTT PCR assays Lower primer of pMUR050 int gene for quantitative real time RT- EcoInt_dw TTACCAACCGAACAGGCTTATG PCR assays Upper primer of V. parahaemolyticus int gene for quantitative real VpaInt_up CTGAATGCTATTTCGTTTTTAT time RT-PCR assays Lower primer of V. parahaemolyticus int gene for quantitative real VpaInt_dw CACCTTTCCCTTGCCAGACT time RT-PCR assays Upper primer of E. coli recA gene for quantitative real time RT-PCR RecAEco_up CCTTGCGGCACGTATGATGA assays Lower primer of E. coli recA gene for quantitative real time RT-PCR RecAEco_dw CACCACGTTTTCGCCCTCTTT assays Upper primer of V. parahaemolyticus recA gene for quantitative RecAVpa_up AAGTAGCATTTCACGCAGTTTG real time RT-PCR assays Lower primer of V. parahaemolyticus recA gene for quantitative RecAVpa_dw AGAAGGCGATGAAGTTGTAGGT real time RT-PCR assays a NdeI or XhoI endonuclease restriction sites included in the oligonucleotide sequences are shown in italics.