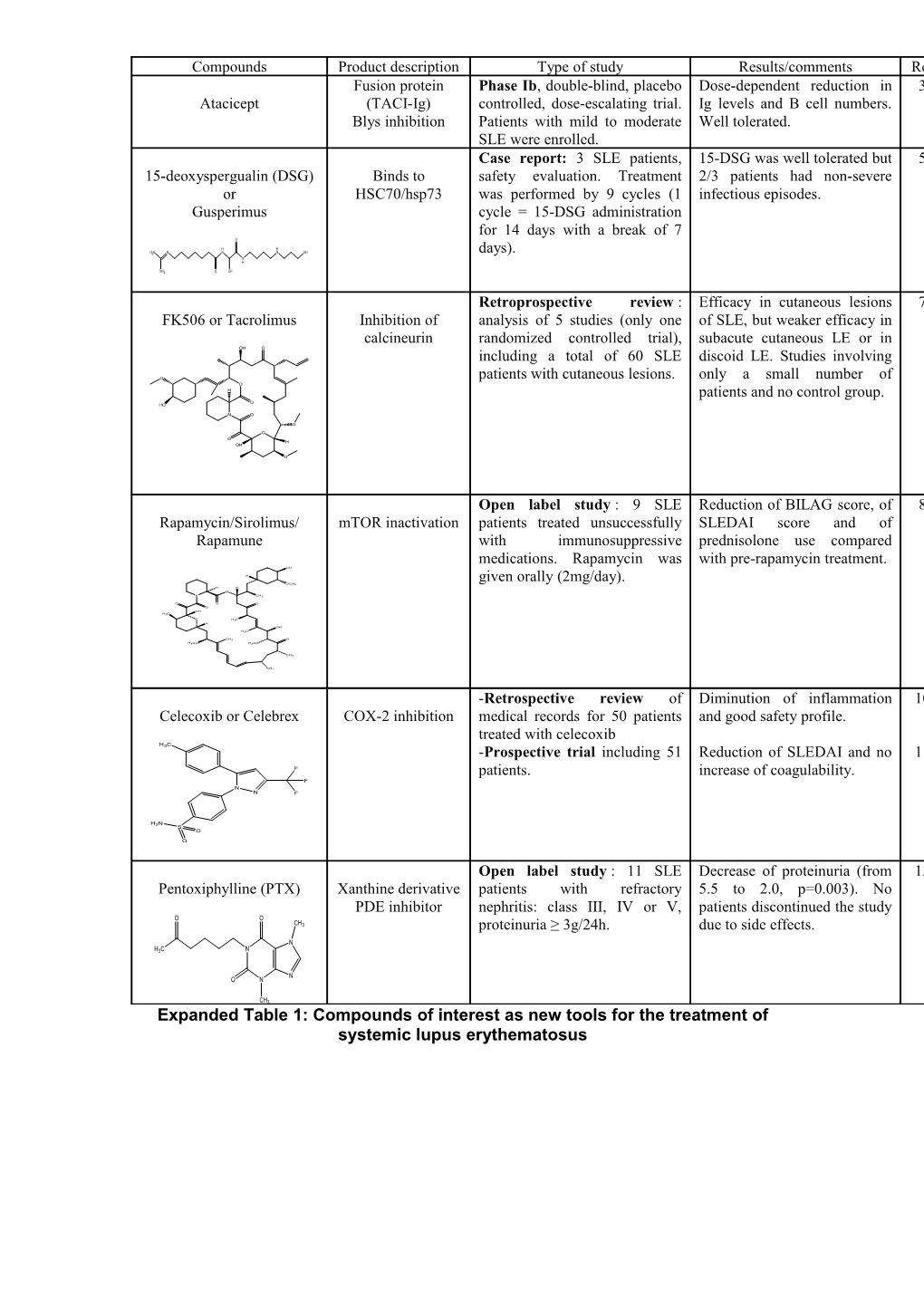
Compounds Product description Type of study Results/comments Ref Fusion protein Phase Ib, double-blind, placebo Dose-dependent reduction in 3 Atacicept (TACI-Ig) controlled, dose-escalating trial. Ig levels and B cell numbers. Blys inhibition Patients with mild to moderate Well tolerated. SLE were enrolled. Case report: 3 SLE patients, 15-DSG was well tolerated but 5 15-deoxyspergualin (DSG) Binds to safety evaluation. Treatment 2/3 patients had non-severe or HSC70/hsp73 was performed by 9 cycles (1 infectious episodes. Gusperimus cycle = 15-DSG administration for 14 days with a break of 7 O
H H H2N N N N NH2 days). N H
NH2 O OH
Retroprospective review : Efficacy in cutaneous lesions 7 FK506 or Tacrolimus Inhibition of analysis of 5 studies (only one of SLE, but weaker efficacy in calcineurin randomized controlled trial), subacute cutaneous LE or in OH O including a total of 60 SLE discoid LE. Studies involving
O patients with cutaneous lesions. only a small number of O H patients and no control group. O HO
N O
O
O O H OH
O
Open label study : 9 SLE Reduction of BILAG score, of 8 Rapamycin/Sirolimus/ mTOR inactivation patients treated unsuccessfully SLEDAI score and of Rapamune with immunosuppressive prednisolone use compared medications. Rapamycin was with pre-rapamycin treatment. OH H given orally (2mg/day). OCH3 H H O
N CH3
O O O O OH H3C
O H3C H OH
H3C
CH3 O H3CO H3CO
CH3
CH3
-Retrospective review of Diminution of inflammation 10 Celecoxib or Celebrex COX-2 inhibition medical records for 50 patients and good safety profile. treated with celecoxib H3C -Prospective trial including 51 Reduction of SLEDAI and no 11 F patients. increase of coagulability. F N N F
H2N S O
O
Open label study : 11 SLE Decrease of proteinuria (from 13 Pentoxiphylline (PTX) Xanthine derivative patients with refractory 5.5 to 2.0, p=0.003). No PDE inhibitor nephritis: class III, IV or V, patients discontinued the study O O CH3 proteinuria ≥ 3g/24h. due to side effects. N H3C N
N O N
CH3 Expanded Table 1: Compounds of interest as new tools for the treatment of systemic lupus erythematosus Double-blind cross-over trial: No improvement of disease 18 Tamoxifen Estrogen antagonist 11 female with stable SLE. activity and 2 patients deteriorated.
H3C O N
CH3 CH3
Review: analysis of randomized -Little clinical effect on 20 DHEA or Prasterone Androgen controlled trials (7) comparing disease activity for patients DHEA to a placebo in SLE with moderate disease. O CH3 patients (842 participants). -Modest but significant improvement in health related CH3 quality of life. -Greater number of participants experiencing HO adverse events.
Double-blind placebo Improvement of SLEDAI but 21 Fulvestrant or Faslodex Estrogen receptor controlled : 20 premenopausal not of serological markers, down regulator SLE women with moderate routine laboratory tests nor OH SLEDAI received either 250 mg bone density. Medications for fulvestran intramuscularly for lupus was reduced in the 12 months (10) or placebo (10). fulvestrant group.
HO (CH2)9SO(CH2)3CF2CF3
-Open label trial : 7 active SLE Serum prolactine and anti- 24 Bromocriptine (BRC) Dopamine agonist patients treated daily during 6 to dsDNA suppressed, SLEDAI inhibition of 9 months. decreased (16 to 5.9). prolactine secretion O -Double-blind, randomized, Significant decreased of 25 N O N O placebo controlled: 66 SLE SLEDAI score (0.9 vs. 2.6 in
O H HO N N H patients (36 BRC, 30 placebo), the control group), decreased H treated daily and followed for 2- of the mean number of
Br 17 months. flares/patient/month (0.08 vs. NH 0.18 in the control group). LJP394/Abetimus Phase III, randomized, Abetimus did not prolong time 27 sodium/Riquent Toleragen placebo controlled trial: 317 i) to renal flare, ii) to initiation molecule; SLE patients with a history of of high-dose corticosteroid 4 strands of ds- renal flares and anti-dsDNA and/or cyclophosphamide oligonucleotides levels >15IU/ml. Patients treatment, or iii) to major SLE (20-mer) linked received 100mg/week for up to flare, but decreased anti- through a 22 months. dsDNA Ab levels (p<0.0001). triethylene glycol based platform
Lupuzor 21-mer peptide Phase IIa: open label, dose Diminution of anti-dsDNA Ab 31 P140 escalating trial. 20 patients with levels and of SLEDAI score in RIHMVYSKRSGKPRGYAFIEY (phosphoserine at moderate SLE were enrolled. the group that received 200µg position 140) Lupuzor was given sc (200µg or of peptide. 1mg).