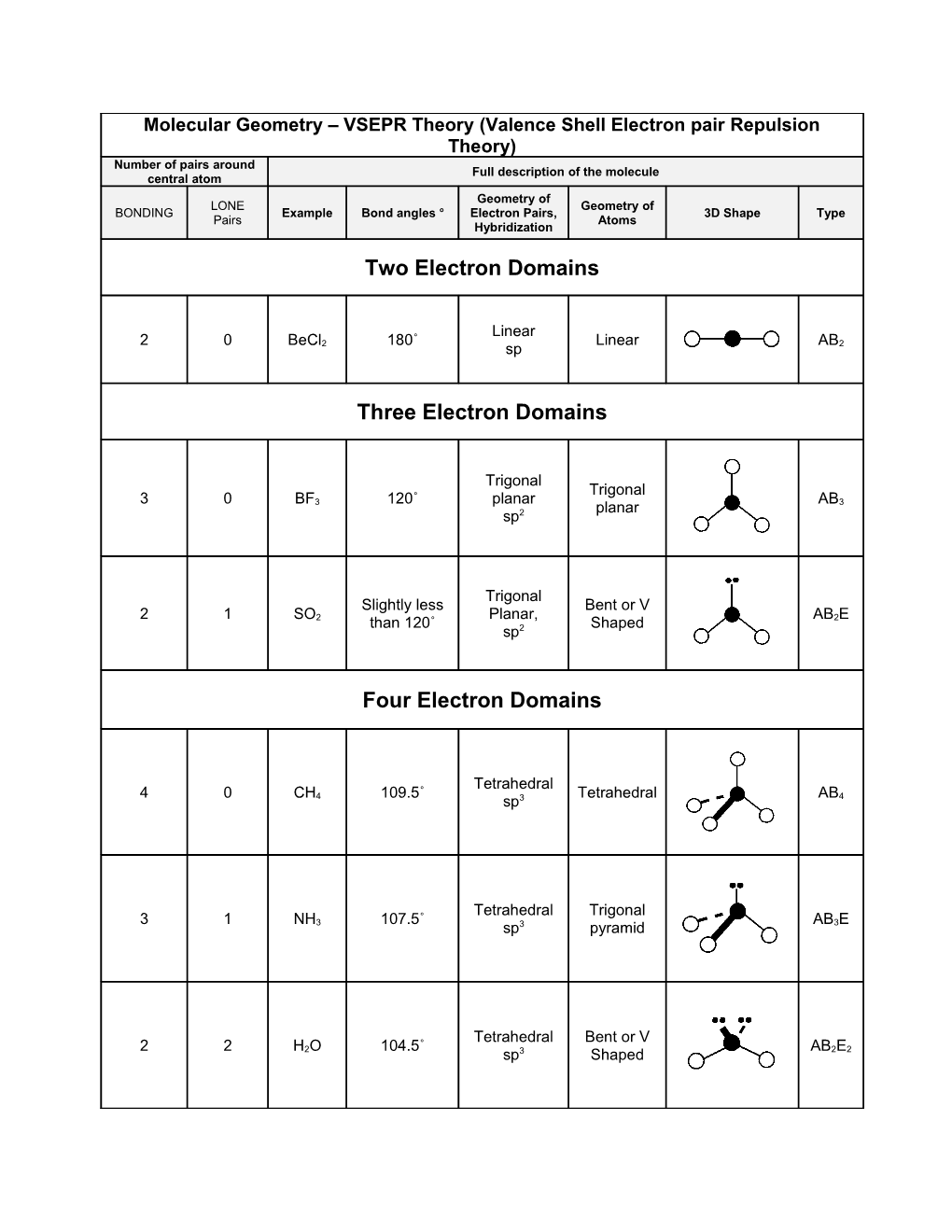
Molecular Geometry – VSEPR Theory (Valence Shell Electron pair Repulsion Theory) Number of pairs around Full description of the molecule central atom Geometry of LONE Geometry of BONDING Example Bond angles o Electron Pairs, 3D Shape Type Pairs Atoms Hybridization
Two Electron Domains
Linear 2 0 BeCl 180˚ Linear AB 2 sp 2
Three Electron Domains
Trigonal Trigonal 3 0 BF 120˚ planar AB 3 planar 3 sp2
Trigonal Slightly less Bent or V 2 1 SO Planar, AB E 2 than 120˚ Shaped 2 sp2
Four Electron Domains
Tetrahedral 4 0 CH 109.5˚ Tetrahedral AB 4 sp3 4
Tetrahedral Trigonal 3 1 NH 107.5˚ AB E 3 sp3 pyramid 3
Tetrahedral Bent or V 2 2 H O 104.5˚ AB E 2 sp3 Shaped 2 2 Geometry of Lone Electron Geometry Bonding Example Bond Angles 3D- Shape Type Pairs pairs, of Atoms Hybridization
Five Electron Domains
120˚ in plane. Trigonal 90˚ Trigonal 5 0 PCl bipyramidal AB 5 perpendicular bipyramidal 5 sp3d to plane
Trigonal 4 1 SF4 Complex bipyramidal Seesaw AB4E sp3d
Trigonal 3 2 ClF3 Approx. 90˚ bipyramidal T-Shaped AB3E2 sp3d
Trigonal 2 3 XeF2 180˚ bipyramidal Linear AB2E3 sp3d
Six Electron Domains
Octahedral 6 0 SF 90˚ Octahedral AB 6 sp3d2 6
Octahedral Square 5 1 BrF 90˚ AB E 5 sp3d2 Pyramidal 5
Octahedral Square 4 2 XeF 90˚ AB E 4 sp3d2 planar 4 2