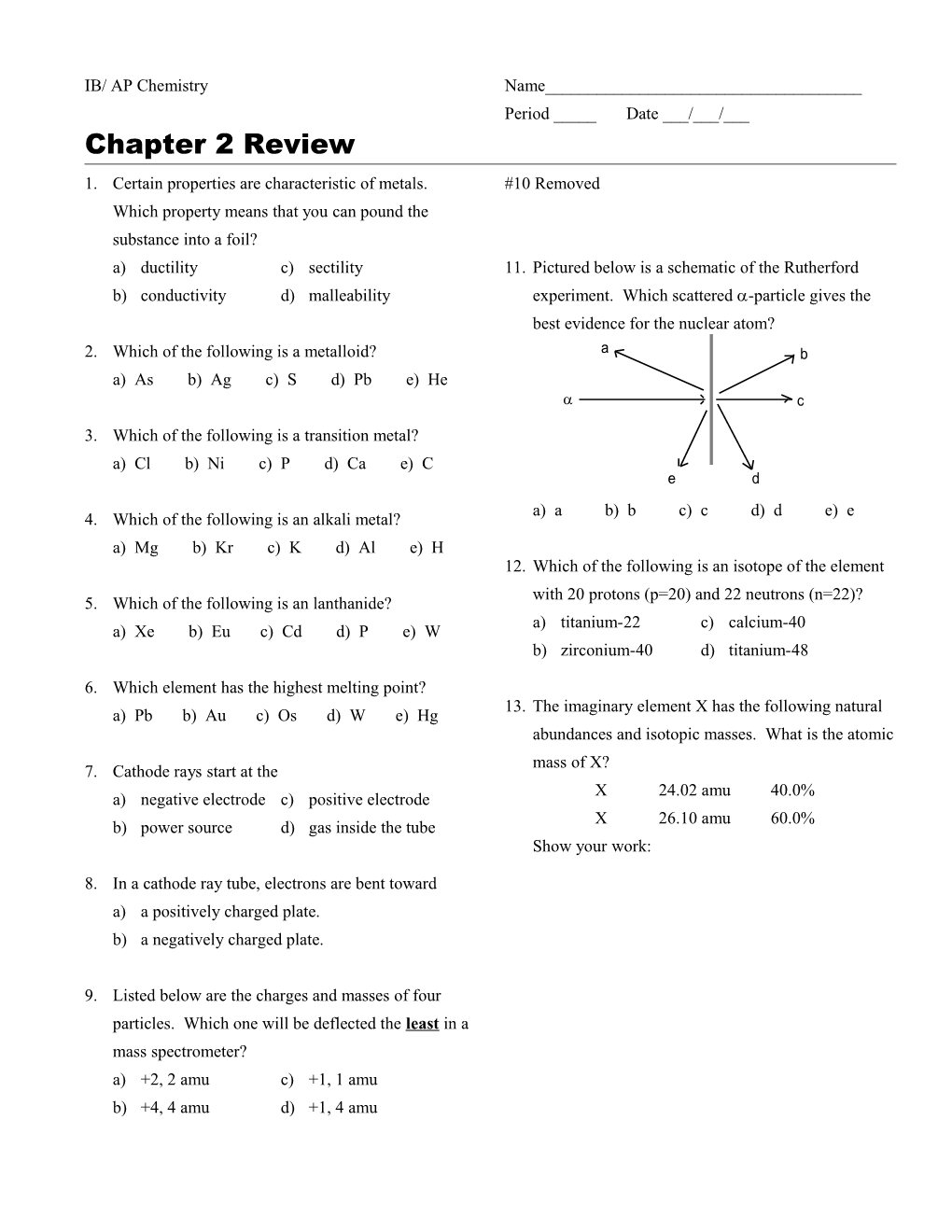
IB/ AP Chemistry Name______Period _____ Date ___/___/___ Chapter 2 Review 1. Certain properties are characteristic of metals. #10 Removed Which property means that you can pound the substance into a foil? a) ductility c) sectility 11. Pictured below is a schematic of the Rutherford b) conductivity d) malleability experiment. Which scattered -particle gives the best evidence for the nuclear atom? 2. Which of the following is a metalloid? a b a) As b) Ag c) S d) Pb e) He c
3. Which of the following is a transition metal? a) Cl b) Ni c) P d) Ca e) C e d a) a b) b c) c d) d e) e 4. Which of the following is an alkali metal? a) Mg b) Kr c) K d) Al e) H 12. Which of the following is an isotope of the element with 20 protons (p=20) and 22 neutrons (n=22)? 5. Which of the following is an lanthanide? a) titanium-22 c) calcium-40 a) Xe b) Eu c) Cd d) P e) W b) zirconium-40 d) titanium-48
6. Which element has the highest melting point? 13. The imaginary element X has the following natural a) Pb b) Au c) Os d) W e) Hg abundances and isotopic masses. What is the atomic mass of X? 7. Cathode rays start at the X 24.02 amu 40.0% a) negative electrode c) positive electrode X 26.10 amu 60.0% b) power source d) gas inside the tube Show your work:
8. In a cathode ray tube, electrons are bent toward a) a positively charged plate. b) a negatively charged plate.
9. Listed below are the charges and masses of four particles. Which one will be deflected the least in a mass spectrometer? a) +2, 2 amu c) +1, 1 amu b) +4, 4 amu d) +1, 4 amu For questions 14 - 17, use the following key: 23. Consider the following notation: Rn (each answer may be used once, more than once, Which statement below is correct? or not at all) a) This particle contains 86 protons a) alpha b) This particle has a mass number of 86 b) beta c) This particle has an atomic number of 220 c) gamma d) This particle contains 220 neutrons d) alpha and beta, but not gamma 24. Which elements did Mendeleev leave spaces for in 14. A high energy form of light his periodic table? 15. Two protons & two neutrons ______16. A high speed electron 17. Used by Ernest Rutherford as a “probe” 25. If copper metal is a mixture two isotopes, Cu-63, mass = 62.9298 u and Cu-65, mass = 64.9278 u. For questions 18 - 22, use the following key: The molar mass of copper is 64.546 g/mole. (each answer may be used once, more than once, Calculate the % abundances of the two isotopes of or not at all.) copper. Show your work. a) John Dalton b) Ernest Rutherford c) J.J. Thomson 26.What is the formula of the ionic compound formed d) Democritus between Mg and Br?
a) MgBr d) Mg2Br2 18. His model of the atom has been called the “plum b) Mg2Br e) Mg2Br3 pudding” Model. c) MgBr2
19. His model of the atom has been called the “billiard 27. What is the formula of the ionic compound formed ball” model. between Ca and P?
a) Ca2P3 d) Ca2P 20. He studied matter in cathode ray tubes. b) CaP e) Ca3P2 c) Ca5P10 21. His philosophical idea included the term “atomos”. 2– 28. What is the name of the SO3 ion? 22. He added to the atomic theory the idea that atoms a) sulfate d) sulfur trioxide had positive and negative parts. b) nitrate e) hydrogen sulfate c) sulfite 29. What is the correct formula and charge for the 36. The correct name of FeO is ______. chromate ion? 2– – a) CrO4 d) Cr2O7 37. The correct name of Cu2O is ______. – 3+ b) CrO4 e) Cr 2– c) Cr2O7 38. The correct name of NiCl2 is ______.
30. Which one of the following elements forms ions 39. The correct formula of barium chloride is _____. with two different valences? a) calcium c) iron 40. The correct formula of silver nitrate is ______. b) arsenic d) fluorine 41. The correct formula of calcium phosphate ______.
31. The correct name for CCl4 is a) carbon(I) chloride 42. The correct condensed structural formula of b) carbon chloride 4-isopropyloctane c) carbon tetrachloride d) monocarbon chloride(IV) e) carbochlorinate 43. The correct condensed structural formula of 4,4-dimethyl-2-pentyne 32. The correct formula for hydrogen telluride is
a) HTe c) H3Te 44. The correct name for the following
b) H2Te d) HTe2
33. The correct formula for dinitrogen tetroxide is a) NO d) NO – 2 3 Just For Fun: b) N O e) (N O) 2 4 2 4 Element names finish these sentences. c) N O 2 5 • A ridiculous inmate is a ___. • I bumped my ___ the car door. 34. The correct name for S Cl is 2 2 • I am sad when all the flowers ____. a) sulfur dichloride • What the police officer does to the b) sulfur(I) chloride crook. ___ c) sulfur(II) chloride • What the doctor does to the patient. d) disulfur dichloride ___ e) sulfur chloride • What the undertaker does if the doctor doesn’t succeed. ___ 35. The correct name for NO2 is • If your cattle get away, ___. a) nitrogen dioxide • A famous London theatre is the ___. b) nitrite • Demonstrations help keep the c) nitrogen oxide lectures from getting ___. • Linoleum, tile, and hardwood are three types of ___.