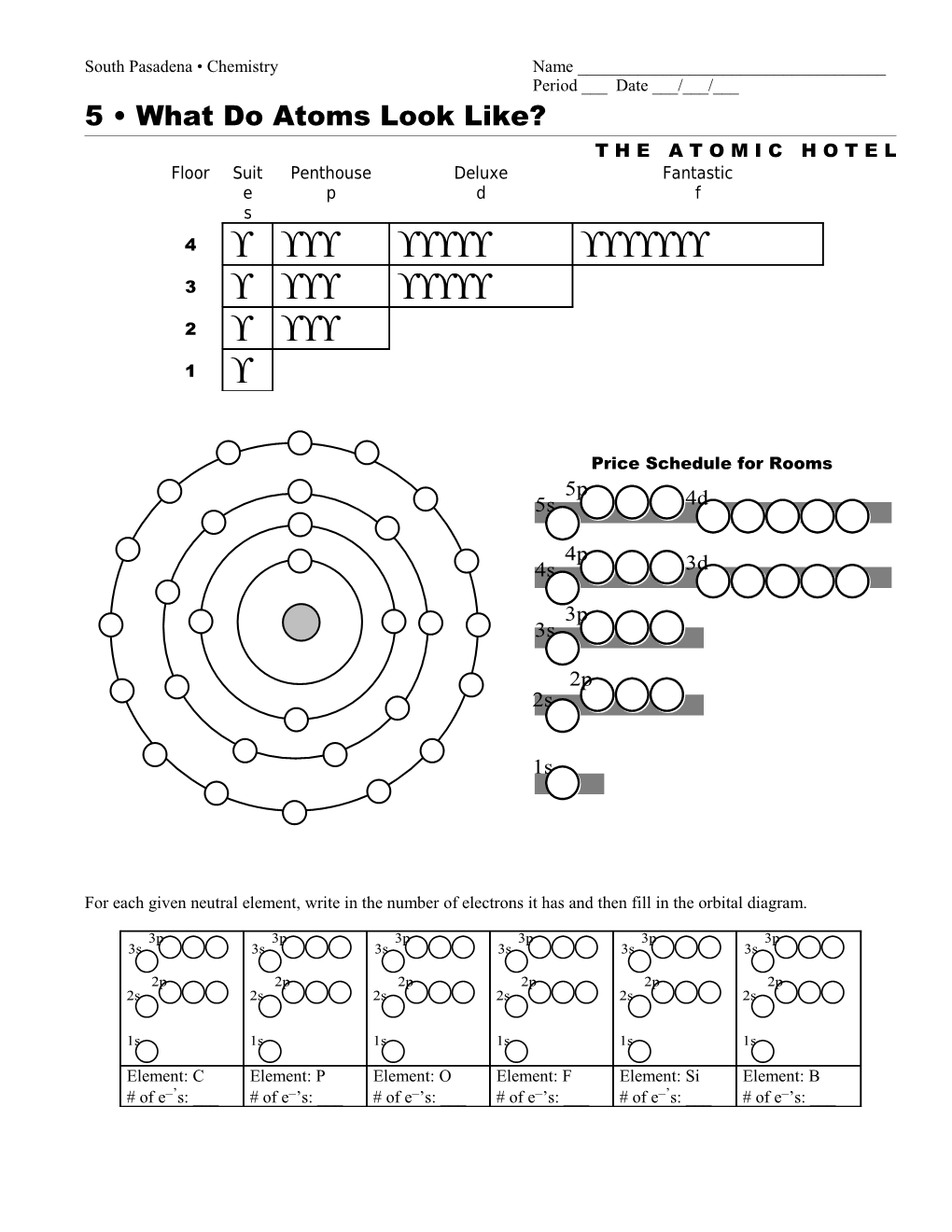
South Pasadena • Chemistry Name ______Period ___ Date ___/___/___ 5 • What Do Atoms Look Like? T H E A T O M I C H O T E L Floor Suit Penthouse Deluxe Fantastic e p d f s 4 3 2 1
Price Schedule for Rooms 5p 5s 4d
4p 4s 3d
3p 3s
2p 2s
1s
For each given neutral element, write in the number of electrons it has and then fill in the orbital diagram.
3p 3p 3p 3p 3p 3p 3s 3s 3s 3s 3s 3s
2p 2p 2p 2p 2p 2p 2s 2s 2s 2s 2s 2s
1s 1s 1s 1s 1s 1s
Element: C Element: P Element: O Element: F Element: Si Element: B # of e–’s: ___ # of e–’s: ___ # of e–’s: ___ # of e–’s: ___ # of e–’s: ___ # of e–’s: ___ Consider the element, Fe with 26 electrons. Fill in the orbital diagram on the left and then fill in the orbitals on the right to match. s p d f 4p 4s 3d 4 3p 3s 3 2p 2s 2
1s 1
If you bumped into an iron atom (Fe), which electron(s) would you bump into? Draw a box around them.
4p 4s 3d Consider 4pthe Oxygen Family: Se Draw the4s orbital diagrams3d for the four members of 3p Family 14: 3s 3p Draw a3s box around the electrons that you would bump into. 2p 2p 2s O 2s 1s 1s 5p Te 5s 4d 3p S 3s 4p 4s 3d 2p 2s 3p 3s
1s 2p 2s
1s
Family Similarities and the Number of Electrons You Would Bump Into: Per 1 2 13 14 15 16 17 18 1 H He 2 Li Be B C N O F Ne 3 Na Mg Al Si P S Cl Ar 4 K Ca Ga Ge As Se Br Kr 5 Rb Sr In Sn Sb Te I Xe 6 Cs Ba Tl Pb Bi Po At Rn 7 Fr Ra