Task 8:Quality Control Plan
Quality Control is defined as the operational techniques and the activities used to keep the quality of inputs or outputs to specifications; to fulfill and verify requirements of quality. It is a part of a good quality management system. The main objective of the Quality Control Plan is to have the right tools and systems to collect the right information to make sure the quality and safety of our products along with the production process, as well as provide possible solutions for on-line monitoring and a well documented trail of the production process.
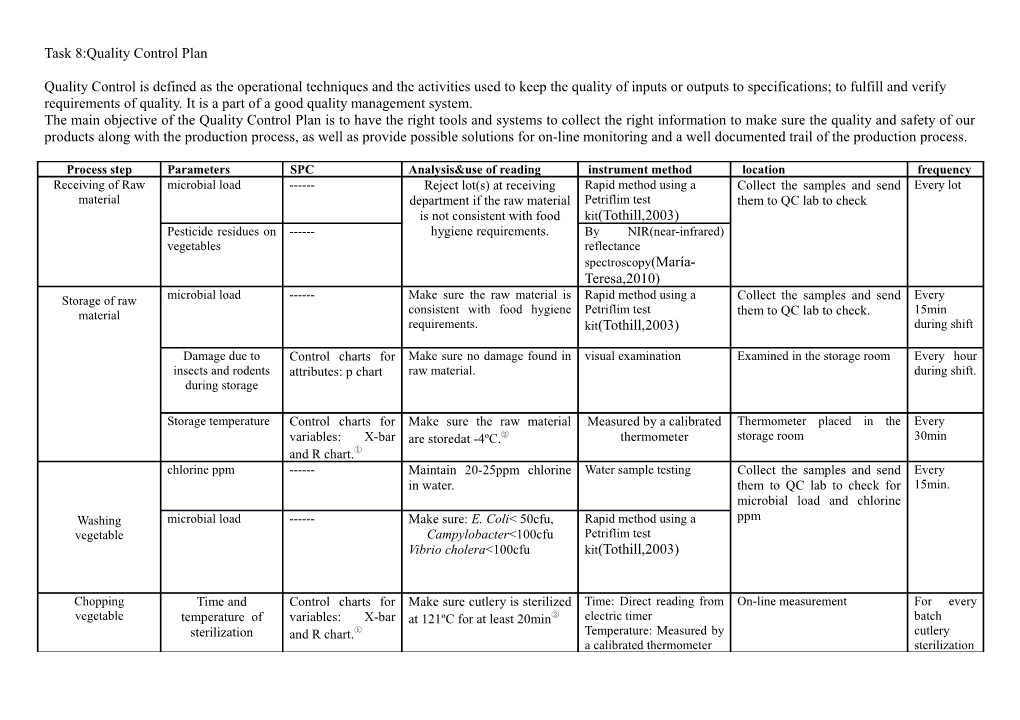
Process step Parameters SPC Analysis&use of reading instrument method location frequency Receiving of Raw microbial load ------Reject lot(s) at receiving Rapid method using a Collect the samples and send Every lot material department if the raw material Petriflim test them to QC lab to check is not consistent with food kit(Tothill,2003) Pesticide residues on ------hygiene requirements. By NIR(near-infrared) vegetables reflectance spectroscopy(María- Teresa,2010) Storage of raw microbial load ------Make sure the raw material is Rapid method using a Collect the samples and send Every material consistent with food hygiene Petriflim test them to QC lab to check. 15min requirements. kit(Tothill,2003) during shift
Damage due to Control charts for Make sure no damage found in visual examination Examined in the storage room Every hour insects and rodents attributes: p chart raw material. during shift. during storage
Storage temperature Control charts for Make sure the raw material Measured by a calibrated Thermometer placed in the Every variables: X-bar are storedat -4ºC.② thermometer storage room 30min and R chart.① chlorine ppm ------Maintain 20-25ppm chlorine Water sample testing Collect the samples and send Every in water. them to QC lab to check for 15min. microbial load and chlorine Washing microbial load ------Make sure: E. Coli< 50cfu, Rapid method using a ppm vegetable Campylobacter<100cfu Petriflim test Vibrio cholera<100cfu kit(Tothill,2003)
Chopping Time and Control charts for Make sure cutlery is sterilized Time: Direct reading from On-line measurement For every vegetable temperature of variables: X-bar at 121ºC for at least 20min② electric timer batch sterilization and R chart.① Temperature: Measured by cutlery a calibrated thermometer sterilization dice size: Diameter Control charts for Make sure cubes of sweet Measured by a vernier Off-line: Collect sample and For every of sweet potato attributes: p chart potato: caliper send to QC lab for microbial batch cubes 5-7mm exam. Time of boiling ------Make sure soya boiled in Direct reading from an On-line measurement: For every Cooking soya water for at least 10min. electric timer measured by an electric timer batch chunks boiled. Cooking filling the time product ------Make sure immediately use Direct reading from On-line measurement: For every was on hold after cooked filling or keep it in electric timer measured by an electric timer batch cooking with and isolated and refrigerated without compartment. refrigeration Dough making properly cleaned and Control charts for Make sure the baffle joint are ProFossTMFlour(a High On-line measurement For every sealed baffle joint attributes: p chart cleaned and sealed properly resolution NIR system for batch flour analysis) microbial load ------Make sure the raw material is Rapid method using a Off-line measurement:stop the every shift consistent with food hygiene Petriflim test line clean machine and start requirements. kit(Tothill,2003) production again and send samples in QC lab for microbial and chemical Forming of samosa analysis. foreign material ------No addition of foreign material By NIR(near-infrared) On-line measurement during stuffing reflectance spectroscopy(María- Teresa,2010) Frying Peroxide value of oil ------Calculate Peroxide value of 1g sample+30ml(glacial: Off-line measurement: sample Every 4 oil, make sure it is less than chloroform::3:2) + 0.5ml is taken from a process line hours 13meq/lit KI(saturated). and measured with a device in (at start and i.e. to check the degree of Add starch indicator. a laboratory. mid of rancidity. Titrate against 0.2N every 8 Na2SO3. hour shift) PV=(Sample- Blank)*N*1000 Wt. of the sample (Bhuyan,2007) Freezing Freezing temperature Control charts for Make sure product be frozen Measured by a calibrated Thermometer placed in the Every variables: X-bar immediately after frying to- thermometer(Kress- freezer 30min and R chart.① 18°C.② Rogers,2001) Packaging Proper 3-seal Control charts for Make sure the products are Visual inspection of On-line measurement Every pack packaging attributes: p chart proper packed packets
Unit weight Control charts for Make sure the weight of each Measured by an On-line measurement Every pack variables: X-bar pack not less than 340g and not electronic scale and R chart.① higher than 348g Metal detection ------No metal should be detected Measured by a metal On-line measurement Every pack detector Storage Storage temperature Control charts for Make sure the products are Measured by a calibrated Thermometer placed in the Every variables: X-bar stored below -18ºC.② thermometer(Kress- storage room 30min and R chart.① Rogers,2001) ① If samples are outside the control limits, determine from the records the assignable causes of the variation ② corrective action needs to be taken when plotted samples fall outside action limits
Quality Control is an approach occurs throughout the production process. It requires performing all activities in conformance with valid requirements. The following performance criteria guarantees this plan can fits into an overall framework for monitoring quality and safety.
This Quality Control plan includes all the details of parameters to measure, control limits of parameters, locations, devices, techniques, frequencies of verification, test methods and a list of equipment that is verified. The Quality Control team have a clear responsibility to ensure all project elements are economical, accurate, properly prepared, coordinated, checked, and completed. The Quality Control team will review plans for compliance with legislation, the product and the production process, good manufacturing practice(GMP), good hygiene practice(GHP) and HACCP plan. The results and analysis of the results of process control calculations will consistent with the data and the food processing quality control situation. Quantity calculations and independent quantity check calculations will be bound and properly identified. All documents with details on control charting, test data, and the diary will be recorded, and all the copies will be kept in form of electronic files.