Solubility Curve Questions
Use the solubility curve below to answer the following questions. For all questions, assume that the density of water is 1.0 g/ mL. Be sure to show all of your calculations.
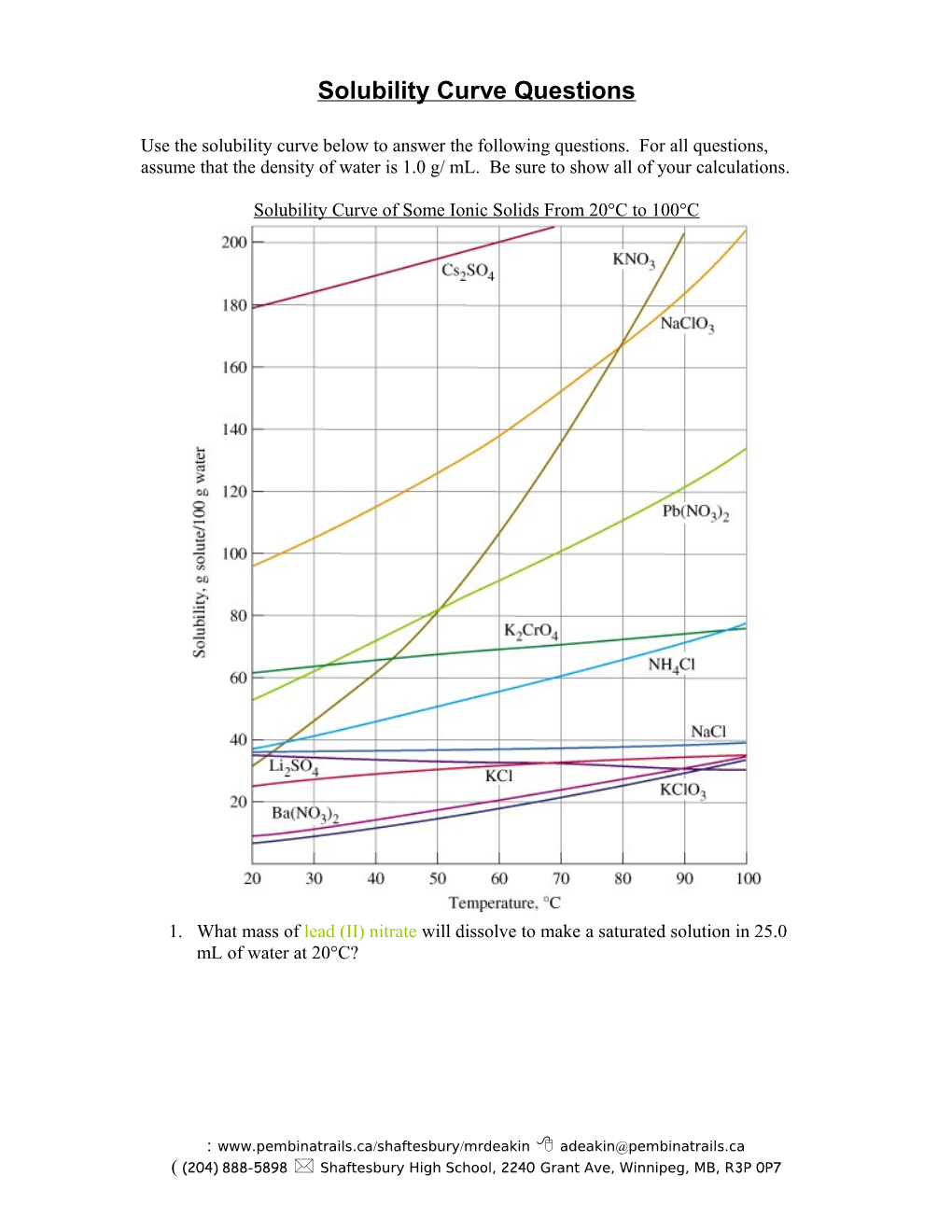
Solubility Curve of Some Ionic Solids From 20°C to 100°C
1. What mass of lead (II) nitrate will dissolve to make a saturated solution in 25.0 mL of water at 20°C?
www.pembinatrails.ca/shaftesbury/mrdeakin [email protected] (204) 888-5898 Shaftesbury High School, 2240 Grant Ave, Winnipeg, MB, R3P 0P7 Solubility Curve Questions
2. What mass of sodium chlorate dissolves 33.3 mL of water at 40°C to make a saturated solution?
3. What mass of lithium sulfate dissolves in 20.0 mL of water at 35°C to make a saturated solution?
4. What mass of potassium chromate precipitates from solution if a saturated solution in 60.0 g of water is cooled from 82°C to 40°C?
5. Why is a precipitate formed in the previous question? 6. What mass of potassium chloride precipitates from a saturated solution in 15 g of water when it is cooled from 90°C to 30°C?
7. A 200.0 mL saturated solution of potassium nitrate is made at 90°C. If 15% of the solvent evaporates as the solution is slowly cooled to 30°C, what mass of precipitate is produced?
www.pembinatrails.ca/shaftesbury/mrdeakin [email protected] (204) 888-5898 Shaftesbury High School, 2240 Grant Ave, Winnipeg, MB, R3P 0P7