Inhibition of Hepatic Cholesterol Synthesis
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

WO 2013/180584 Al 5 December 2013 (05.12.2013) P O P C T
(12) INTERNATIONAL APPLICATION PUBLISHED UNDER THE PATENT COOPERATION TREATY (PCT) (19) World Intellectual Property Organization International Bureau (10) International Publication Number (43) International Publication Date WO 2013/180584 Al 5 December 2013 (05.12.2013) P O P C T (51) International Patent Classification: AO, AT, AU, AZ, BA, BB, BG, BH, BN, BR, BW, BY, C12N 1/21 (2006.01) C12N 15/74 (2006.01) BZ, CA, CH, CL, CN, CO, CR, CU, CZ, DE, DK, DM, C12N 15/52 (2006.01) C12P 5/02 (2006.01) DO, DZ, EC, EE, EG, ES, FI, GB, GD, GE, GH, GM, GT, C12N 15/63 (2006.01) HN, HR, HU, ID, IL, IN, IS, JP, KE, KG, KN, KP, KR, KZ, LA, LC, LK, LR, LS, LT, LU, LY, MA, MD, ME, (21) International Application Number: MG, MK, MN, MW, MX, MY, MZ, NA, NG, NI, NO, NZ, PCT/NZ20 13/000095 OM, PA, PE, PG, PH, PL, PT, QA, RO, RS, RU, RW, SC, (22) International Filing Date: SD, SE, SG, SK, SL, SM, ST, SV, SY, TH, TJ, TM, TN, 4 June 2013 (04.06.2013) TR, TT, TZ, UA, UG, US, UZ, VC, VN, ZA, ZM, ZW. (25) Filing Language: English (84) Designated States (unless otherwise indicated, for every kind of regional protection available): ARIPO (BW, GH, (26) Publication Language: English GM, KE, LR, LS, MW, MZ, NA, RW, SD, SL, SZ, TZ, (30) Priority Data: UG, ZM, ZW), Eurasian (AM, AZ, BY, KG, KZ, RU, TJ, 61/654,412 1 June 2012 (01 .06.2012) US TM), European (AL, AT, BE, BG, CH, CY, CZ, DE, DK, EE, ES, FI, FR, GB, GR, HR, HU, IE, IS, IT, LT, LU, LV, (71) Applicant: LANZATECH NEW ZEALAND LIMITED MC, MK, MT, NL, NO, PL, PT, RO, RS, SE, SI, SK, SM, [NZ/NZ]; 24 Balfour Road, Parnell, Auckland, 1052 (NZ). -

S-Abscisic Acid
CLH REPORT FOR[S-(Z,E)]-5-(1-HYDROXY-2,6,6-TRIMETHYL-4-OXOCYCLOHEX-2-EN- 1-YL)-3-METHYLPENTA-2,4-DIENOIC ACID; S-ABSCISIC ACID CLH report Proposal for Harmonised Classification and Labelling Based on Regulation (EC) No 1272/2008 (CLP Regulation), Annex VI, Part 2 International Chemical Identification: [S-(Z,E)]-5-(1-hydroxy-2,6,6-trimethyl-4-oxocyclohex-2- en-1-yl)-3-methylpenta-2,4-dienoic acid; S-abscisic acid EC Number: 244-319-5 CAS Number: 21293-29-8 Index Number: - Contact details for dossier submitter: Bureau REACH National Institute for Public Health and the Environment (RIVM) The Netherlands [email protected] Version number: 1 Date: August 2018 Note on confidential information Please be aware that this report is intended to be made publicly available. Therefore it should not contain any confidential information. Such information should be provided in a separate confidential Annex to this report, clearly marked as such. [04.01-MF-003.01] CLH REPORT FOR[S-(Z,E)]-5-(1-HYDROXY-2,6,6-TRIMETHYL-4-OXOCYCLOHEX-2-EN- 1-YL)-3-METHYLPENTA-2,4-DIENOIC ACID; S-ABSCISIC ACID CONTENTS 1 IDENTITY OF THE SUBSTANCE........................................................................................................................1 1.1 NAME AND OTHER IDENTIFIERS OF THE SUBSTANCE...............................................................................................1 1.2 COMPOSITION OF THE SUBSTANCE..........................................................................................................................1 2 PROPOSED HARMONISED -

Relationship to Atherosclerosis
AN ABSTRACT OF THE THESIS OF Marilyn L. Walsh for the degree of Doctor of Philosophy in Biochemistry and Biophysics presented on May 3..2001. Title: Protocols. Pathways. Peptides and Redacted for Privacy Wilbert Gamble The vascular system transports components essential to the survival of the individual and acts as a bamer to substances that may injure the organism. Atherosclerosis is a dynamic, lesion producing disease of the arterial system that compromises the functioning of the organ by occlusive and thrombogenic processes. This investigation was undertaken to elucidate some of the normal biochemical processes related to the development of atherosclerosis. A significant part of the investigation was directed toward developing and combining methods and protocols to obtain the data in a concerted manner. A postmitochondnal supernatant of bovine aorta, usingmevalonate-2-14C as the substrate, was employed in the investigation. Methods included paper, thin layer, and silica gel chromatography; gel filtration, high performance liquid chromatography (HPLC), and mass spectrometry. This current research demonstrated direct incorporation of mevalonate-2- 14Cinto the trans-methyiglutaconic shunt intermediates. The aorta also contains alcohol dehydrogenase activity, which converts dimethylallyl alcohol and isopentenol to dimethylacrylic acid, a constituent of the trans-methylgiutaconate Small, radioactive peptides, named Nketewa as a group, were biosynthesized using mevalonate-2-'4C as the substrate. They were shown to pass through a 1000 D membrane. Acid hydrolysis and dabsyl-HPLC analysis defined the composition of the Nketewa peptides. One such peptide, Nketewa 1, had a molecular weight of 1038 and a sequence of his-gly-val-cys-phe-ala-ser-met (HGVCFASM), with afarnesyl group linked via thioether linkage to the cysteine residue. -

Plasma Mevalonate As a Measure of Cholesterol Synthesis in Man
Plasma mevalonate as a measure of cholesterol synthesis in man. T S Parker, … , J Chen, P J De Schepper J Clin Invest. 1984;74(3):795-804. https://doi.org/10.1172/JCI111495. Research Article Measurement of mevalonic acid (MVA) concentrations in plasma or 24-h urine samples is shown to be useful in studies of the regulation of 3-hydroxy-3-methylglutaryl coenzyme A (HMG-CoA) reductase and cholesterol synthesis. Plasma MVA concentrations, measured either at 7-9 a.m. after an overnight fast, or throughout the 24-h cycle, were compared with cholesterol synthesis rates that were measured by the sterol balance method: plasma MVA concentrations were directly related to the rate of whole body cholesterol synthesis (r = 0.972; p less than 0.001; n = 18) over a tenfold range of cholesterol synthesis rates. Moreover, hourly examination of MVA concentrations throughout the day demonstrated that interventions such as fasting or cholesterol feeding cause suppression of the postmidnight diurnal rise in plasma MVA concentrations, with little change in the base-line of the rhythm. Thus, the daily rise and fall of plasma MVA appears to reflect changes in tissues and organs, such as the liver and intestine, that are known to be most sensitive to regulation by fasting or by dietary cholesterol. The hypothesis that short-term regulation of HMG-CoA reductase in tissues is quickly reflected by corresponding variations in plasma MVA was tested by using a specific inhibitor of HMG-CoA reductase, mevinolin, to block MVA synthesis. Mevinolin caused a dose-dependent lowering of plasma MVA after a single dose; and in patients who […] Find the latest version: https://jci.me/111495/pdf Plasma Mevalonate as a Measure of Cholesterol Synthesis in Man Thomas S. -

Medically Useful Plant Terpenoids: Biosynthesis, Occurrence, and Mechanism of Action
molecules Review Medically Useful Plant Terpenoids: Biosynthesis, Occurrence, and Mechanism of Action Matthew E. Bergman 1 , Benjamin Davis 1 and Michael A. Phillips 1,2,* 1 Department of Cellular and Systems Biology, University of Toronto, Toronto, ON M5S 3G5, Canada; [email protected] (M.E.B.); [email protected] (B.D.) 2 Department of Biology, University of Toronto–Mississauga, Mississauga, ON L5L 1C6, Canada * Correspondence: [email protected]; Tel.: +1-905-569-4848 Academic Editors: Ewa Swiezewska, Liliana Surmacz and Bernhard Loll Received: 3 October 2019; Accepted: 30 October 2019; Published: 1 November 2019 Abstract: Specialized plant terpenoids have found fortuitous uses in medicine due to their evolutionary and biochemical selection for biological activity in animals. However, these highly functionalized natural products are produced through complex biosynthetic pathways for which we have a complete understanding in only a few cases. Here we review some of the most effective and promising plant terpenoids that are currently used in medicine and medical research and provide updates on their biosynthesis, natural occurrence, and mechanism of action in the body. This includes pharmacologically useful plastidic terpenoids such as p-menthane monoterpenoids, cannabinoids, paclitaxel (taxol®), and ingenol mebutate which are derived from the 2-C-methyl-d-erythritol-4-phosphate (MEP) pathway, as well as cytosolic terpenoids such as thapsigargin and artemisinin produced through the mevalonate (MVA) pathway. We further provide a review of the MEP and MVA precursor pathways which supply the carbon skeletons for the downstream transformations yielding these medically significant natural products. Keywords: isoprenoids; plant natural products; terpenoid biosynthesis; medicinal plants; terpene synthases; cytochrome P450s 1. -

European Patent Office
(19) & (11) EP 2 380 989 A1 (12) EUROPEAN PATENT APPLICATION published in accordance with Art. 153(4) EPC (43) Date of publication: (51) Int Cl.: 26.10.2011 Bulletin 2011/43 C12Q 1/26 (2006.01) C12N 1/20 (2006.01) C12N 9/02 (2006.01) (21) Application number: 10731334.8 (86) International application number: (22) Date of filing: 19.01.2010 PCT/JP2010/050565 (87) International publication number: WO 2010/082665 (22.07.2010 Gazette 2010/29) (84) Designated Contracting States: (72) Inventor: MATSUOKA, Takeshi AT BE BG CH CY CZ DE DK EE ES FI FR GB GR Tokyo 101-8101 (JP) HR HU IE IS IT LI LT LU LV MC MK MT NL NO PL PT RO SE SI SK SM TR (74) Representative: Forstmeyer, Dietmar et al BOETERS & LIECK (30) Priority: 19.01.2009 JP 2009009177 Oberanger 32 80331 München (DE) (71) Applicant: Asahi Kasei Pharma Corporation Tokyo 101-8101 (JP) (54) METHOD AND REAGENT FOR DETERMINING MEVALONIC ACID, 3- HYDROXYMETHYLGLUTARYL-COENZYME A AND COENZYME A (57) The present invention provides a method for taryl coenzyme A in the presence of a hydrogen acceptor measuring the concentration of an analyte in a test so- X, a hydrogen donor Y, and coenzyme A; and (q) a step lution wherein the analyte is mevalonic acid and/or 3- of measuring an amount of: a reduced hydrogen acceptor hydroxymethylglutaryl coenzyme A, comprising the fol- X that is produced; or an oxidized hydrogen donor Y that lowing steps (p) and (q): (p) a step of allowing an enzyme is produced; or a hydrogen acceptor X that is decreased; that catalyzes a reaction represented by Reaction For- or a hydrogen donor Y that is decreased, wherein the mula 1 and an enzyme that catalyzes a reaction repre- hydrogen donor Y and the reduced hydrogen acceptor sented by Reaction Formula 2 to act on a test solution X are not the same. -

Carboxylic Acids
13 Carboxylic Acids The active ingredients in these two nonprescription pain relievers are derivatives of arylpropanoic acids. See Chemical Connections 13A, “From Willow Bark to Aspirin and Beyond.” Inset: A model of (S)-ibuprofen. (Charles D. Winters) KEY QUESTIONS 13.1 What Are Carboxylic Acids? HOW TO 13.2 How Are Carboxylic Acids Named? 13.1 How to Predict the Product of a Fischer 13.3 What Are the Physical Properties of Esterification Carboxylic Acids? 13.2 How to Predict the Product of a B-Decarboxylation 13.4 What Are the Acid–Base Properties of Reaction Carboxylic Acids? 13.5 How Are Carboxyl Groups Reduced? CHEMICAL CONNECTIONS 13.6 What Is Fischer Esterification? 13A From Willow Bark to Aspirin and Beyond 13.7 What Are Acid Chlorides? 13B Esters as Flavoring Agents 13.8 What Is Decarboxylation? 13C Ketone Bodies and Diabetes CARBOXYLIC ACIDS ARE another class of organic compounds containing the carbonyl group. Their occurrence in nature is widespread, and they are important components of foodstuffs such as vinegar, butter, and vegetable oils. The most important chemical property of carboxylic acids is their acidity. Furthermore, carboxylic acids form numerous important derivatives, including es- ters, amides, anhydrides, and acid halides. In this chapter, we study carboxylic acids themselves; in Chapters 14 and 15, we study their derivatives. 457 458 CHAPTER 13 Carboxylic Acids 13.1 What Are Carboxylic Acids? Carboxyl group A J COOH The functional group of a carboxylic acid is a carboxyl group, so named because it is made group. up of a carbonyl group and a hydroxyl group (Section 1.7D). -

Cholesterol Homeostasis
FOR LIFE SCIENCE RESEARCH Volume 2 Number 7 Cholesterol Homeostasis ■ HMGR Assay Kit ■ Cholesterol Biosynthesis ■ Blocking Absorption of Dietary Cholesterol Hypercholesterolemia can lead to ■ Cholesterol Esterification the formation of plaques and the development of atherosclerosis. ■ Cholesterol Transport ■ Bile Acids Lipid Resource Sigma has now concentrated all its lipids and lipid related products into one easy-to-navigate location. Quickly find the specific lipids you need from over 2,000: ■ Fatty Acids ■ Sphingolipids ■ Glycerides ■ Prenols ■ Complex Lipids ■ Fluorescent Labeled Lipids ■ Oils ■ Analytical Standards ■ Bioactive Lipids Browse for lipids in cell signaling using our interactive pathway charts. Discover everything researchers need for lipid research at sigma.com/lipids. sigma-aldrich.com 1 Introduction Cholesterol is an essential biological molecule that performs many functions within the body. It is a structural component of all cell membranes and is also a precursor to steroid hormones, vitamin D, and bile acids that aid in digestion. Within membranes FOR LIFE SCIENCE RESEARCH the cholesterol to polar lipid ratios affect stability, permeability, and protein mobility. The hormones produced from cholesterol include androgens, estrogens, and the gluco- and 2007 mineralocorticoids. Volume 2 Cholesterol levels in the body are achieved via two sources. Adults with healthy diets will biosynthesize the majority of their cholesterol in the liver and other body tissues Number 7 and obtain the remainder from the dietary intake of foods high in saturated fatty acids. Free cholesterol is not found in blood; rather it is esterified to fatty acids and packaged in lipoprotein particles. Very low density lipoproteins (VLDL) are produced by Table of Contents the liver and consist of an outer core composed of apolipoproteins; apo-B100, apo-CI, apo-CII, apo-CIII, and apoE surrounding an inner core of phospholipids, triglycerides, cholesterol, and cholesteryl esters. -

Mevalonic Acid in Human Plasma
Proc. NatL Acad. Sci. USA Vol. 79, pp. 3037-3041,- May 1982 Medical Sciences Mevalonic acid in human plasma: Relationship of concentration and circadian rhythm to cholesterol synthesis rates in man (sterol balance/cholestyramine resin/fasting/mononuclear leukocytes/dietary cholesterol) THOMAS S. PARKER*, DONALD J. MCNAMARA*, CLINTON BROWN*, OWEN GARRIGANt, RACHAEL KOLB*, HEDDA BATwIN*, AND E. H. AHRENS, JR.* *The Rockefeller University, New York, New York 10021; and tThe Department of Chemistry, Seton Hall University, South Orange, New Jersey 07079 Contributed by Edward H. Ahrens, Jr., February 1, 1982 ABSTRACT We tested the hypothesis that the rate of choles- estimating cholesterol synthesis rates in human subjects with- terol synthesis in tissues determines the concentrations of meva- out the need for in vivo administration ofradioactive materials. Ionic acid (MVA) in plasma. We found that plasma MVA concen- trations were correlated (i) with increased rates of whole-body cholesterol synthesis (measured by sterol-balance methods) in pa- MATERIALS AND METHODS tients treated with cholestyramine resin and (ii) with decreased Out-Patient Volunteers and Their Diets. Ten male patients rates of whole-body sterol synthesis (indicated by conversion of were recruited from the Center for Prevention of Premature labeled acetate to sterol in freshly isolated mononuclear leuko- Arteriosclerosis at the Rockefeller University Hospital from a cytes) in out-patients after 4 weeks on a cholesterol-rich diet. In group of 150 patients previously described (10). Clinical- data addition, a diurnal rhythm of plasma MVA concentrations was and lipid phenotypes for the group are given in Table 1. These observed in patients whose activities were strictly controlled on patients had no symptoms ofischemic heart disease, gallbladder a metabolic ward. -

Mevastatin in Colon Cancer by Spectroscopic and Microscopic Methods - Raman Imaging and AFM Studies
bioRxiv preprint doi: https://doi.org/10.1101/2021.08.31.458425; this version posted September 1, 2021. The copyright holder for this preprint (which was not certified by peer review) is the author/funder. All rights reserved. No reuse allowed without permission. Mevastatin in colon cancer by spectroscopic and microscopic methods - Raman imaging and AFM studies K. Beton1*, P. Wysocki1, B. Brozek-Pluska1* Lodz University of Technology, Institute of Applied Radiation Chemistry, Laboratory of Laser Molecular Spectroscopy, Wroblewskiego 15, 93-590 Lodz and Poland *Correspondence: [email protected]; Tel.: +48 42 631 31 65, [email protected]; Tel.: +48 42 631 31 65 Abstract One of the most important areas of medical science is oncology, which is responsible for both the diagnostics and treatment of cancer diseases. Simultaneously one of the main challenges of oncology is the development of modern drugs effective in the fight against cancer. Statins are a group of biologically active compounds with the activity of 3-hydroxy-3- methyl glutaryl-CoA reductase inhibitors, an enzyme catalyzing the reduction of 3-hydroxy-3- methyl-glutaryl-CoA (HMG-CoA) to mevalonic acid. By acting on this enzyme, statins inhibit the endogenous cholesterol synthesis which in turn causes the reduction of its systemic concentrations. However, in vitro and in vivo studies confirm also the cytostatic and cytotoxic effects of statins against various types of cancer cells including colon cancer. In the presented studies the influence of mevastatin on cancerous colon cells CaCo-2 by Raman spectroscopy and imaging is discussed and compared with biochemistry characteristic for normal colon cells CCD-18Co. -

Role of the Kidneys in the Metabolism of Plasma Mevalonate. Studies in Humans and in Rhesus Monkeys
Role of the kidneys in the metabolism of plasma mevalonate. Studies in humans and in rhesus monkeys. D J McNamara, … , T S Parker, K Morrissey J Clin Invest. 1985;76(1):31-39. https://doi.org/10.1172/JCI111962. Research Article Studies were carried out in humans and in rhesus monkeys to determine the role of the kidneys in the metabolism of circulating mevalonic acid (MVA). Following intravenous infusion of [14C]MVA and [3H]cholesterol, there was a rapid appearance of [14C]squalene in the kidneys that exhibited a significantly longer half-life than plasma or hepatic squalene. In man and in rhesus monkeys there was a rapid equilibration between newly synthesized cholesterol from MVA and exogenously administered cholesterol in all tissues except the kidneys, where the specific activity ratio of newly synthesized to exogenous cholesterol was significantly higher. Estimates of the quantitative metabolism of intravenously infused radiolabeled MVA in the monkey demonstrated that 23% was excreted in the urine, 67% metabolized to cholesterol (58% in nonrenal tissues and 9% in the kidneys), and 10% catabolized to CO2 and nonsteroid products. Measurements of MVA metabolism in anephric and uninephric patients demonstrate that, in the absence of renal uptake of MVA, exogenous and newly synthesized cholesterol achieve almost instantaneous equilibrium in the plasma; whereas in control subjects with normal renal function, this equilibration required at least 21 d for the two cholesterol decay curves to become parallel. These results suggest that the kidneys are solely responsible for the observed disequilibrium between newly synthesized and exogenous cholesterol; we suggest that this was due to the delayed release […] Find the latest version: https://jci.me/111962/pdf Role of the Kidneys in the Metabolism of Plasma Mevalonate Studies in Humans and in Rhesus Monkeys Donald J. -
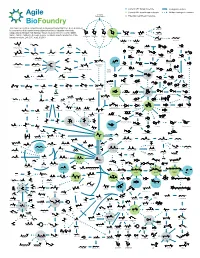
ABF Metabolic Map 15
Current ABF target molecule Biological reaction Current ABF beachhead molecule Multiple biological reactions Biomass Polysaccharides Potential beachhead molecule HO This map represents a compilation of metabolic pathways that have been described OH HO HO Glycolic acid HO HO HO O OH O for conversion of biomass-derived polysaccharides to industrial chemicals. OH OOH O O O OH O OH OH OH HO HO OH OH Adapted by permission from Springer Nature Customer Service Centre GmbH: OH OH OH OH OH Ethylene glycol Nature, Nature Catalysis, A comprehensive metabolic map for production of bio- OH OH OH OH HO OH OH L-Arabinose Galactose Glucose D-Xylose D-xylono-1,4-lactone Xylonic acid OH OH OH based chemicals, Lee, S.Y., et al., © 2019 HO OH OH OH OH HO HO OH HO OH 1,2,4-Butanetriol 1,4-Butanediol O OH OH OH Xylitol D-Arabinitol HO HO O O O HO HO NH2 CoA OH S OH Propanol OH Lactaldehyde 4-Hydroxyhexan-3-one HO OH Malonyl-CoA HO Benzyl alcohol Resveratrol Hydroxytyrosol HO - O O O CoA S O O OH -O O O N HO OH OH O O OH H O O OH HO Benzene O 4-phenylbutyrate O Hippurate Acetol Methylglyoxal Ferulic acid p-Coumaroyl-CoA OH O Propanal 1,2-Propanediol HO 4-Hydroxy-2-oxohexanoic acid p-Hydroxystyrene HO NH2 Benzaldehyde HO OH OH OH Dopamine O OH 1,3-Propanediol O O O O O P - OH O HO O O HO HO HO Glycerone HO OH OH O HO HO 6-phenylhexanoate OH cis, cis-Muconic acid HO O OH O Glyceraldehyde OH HO OH OH HO O O O P - Benzoic acid 3-Phosphate Dihydrobenzenediol Caffeic acid p-Coumaric acid O OH HO 1,3-propanediol 3-Hydroxypropanal HO O HO OH HO NH Glycerol O 2 OH