Commonly Performed Lab Tests and Procedures (Not all-inclusive)
If you need tests that are not listed below, please contact the Division of Laboratory Services at 502-564-4446.
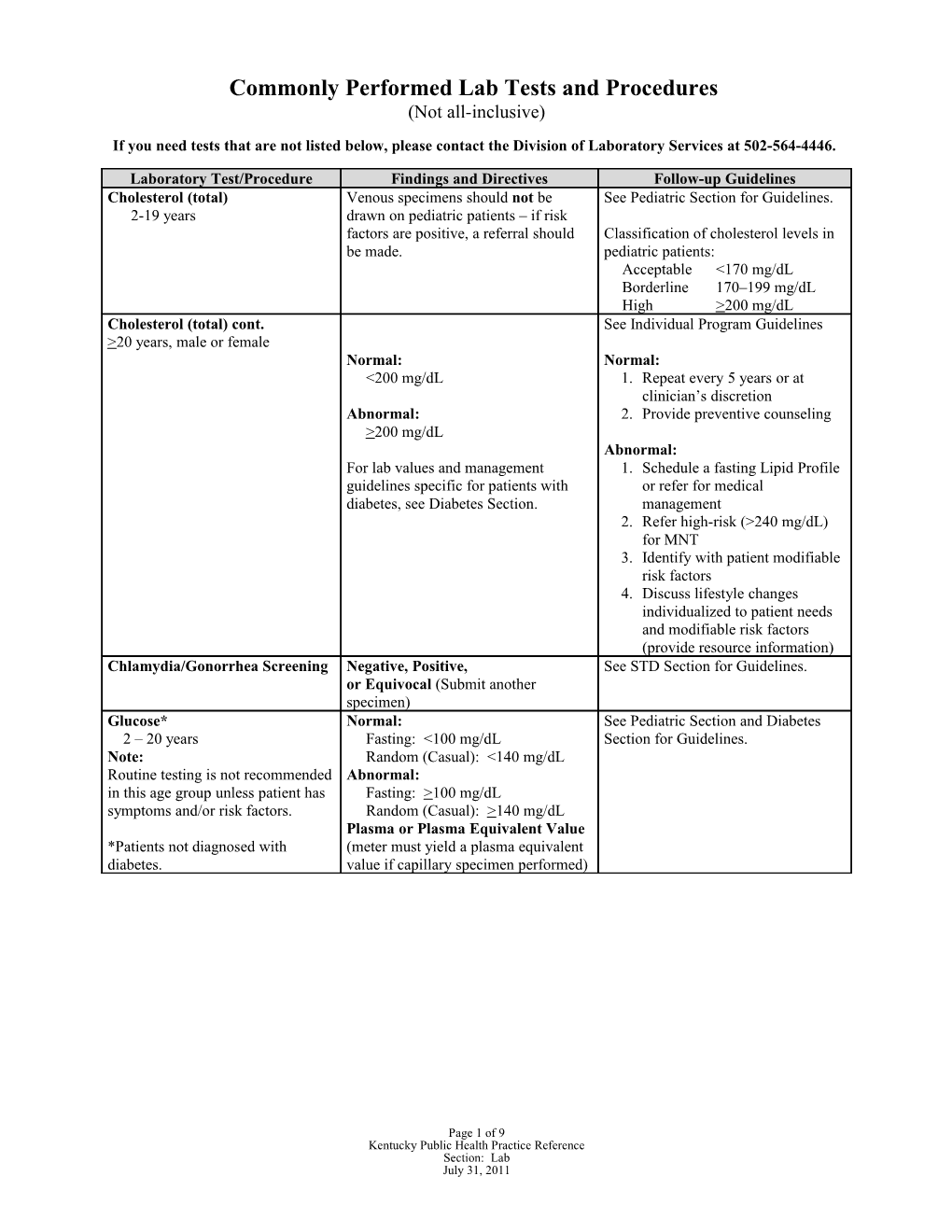
Laboratory Test/Procedure Findings and Directives Follow-up Guidelines Cholesterol (total) Venous specimens should not be See Pediatric Section for Guidelines. 2-19 years drawn on pediatric patients – if risk factors are positive, a referral should Classification of cholesterol levels in be made. pediatric patients: Acceptable <170 mg/dL Borderline 170–199 mg/dL High >200 mg/dL Cholesterol (total) cont. See Individual Program Guidelines >20 years, male or female Normal: Normal: <200 mg/dL 1. Repeat every 5 years or at clinician’s discretion Abnormal: 2. Provide preventive counseling >200 mg/dL Abnormal: For lab values and management 1. Schedule a fasting Lipid Profile guidelines specific for patients with or refer for medical diabetes, see Diabetes Section. management 2. Refer high-risk (>240 mg/dL) for MNT 3. Identify with patient modifiable risk factors 4. Discuss lifestyle changes individualized to patient needs and modifiable risk factors (provide resource information) Chlamydia/Gonorrhea Screening Negative, Positive, See STD Section for Guidelines. or Equivocal (Submit another specimen) Glucose* Normal: See Pediatric Section and Diabetes 2 – 20 years Fasting: <100 mg/dL Section for Guidelines. Note: Random (Casual): <140 mg/dL Routine testing is not recommended Abnormal: in this age group unless patient has Fasting: >100 mg/dL symptoms and/or risk factors. Random (Casual): >140 mg/dL Plasma or Plasma Equivalent Value *Patients not diagnosed with (meter must yield a plasma equivalent diabetes. value if capillary specimen performed)
Page 1 of 9 Kentucky Public Health Practice Reference Section: Lab July 31, 2011 Commonly Performed Lab Tests and Procedures continued (Not all-inclusive)
Laboratory Test/Procedure Findings and Directives Follow-up Guidelines Glucose - Gestational 50 gm Oral Glucose Load See Gestational Diabetes Guidelines in the 1 hour after 50 gm load Plasma Prenatal Section. Glucose Normal: <140 mg/dL
The Glucose Tolerance Test (GTT) 100 gm Glucose Tolerance Test should be performed in the Diagnostic Criteria: Two or more morning after an overnight fast of of the venous plasma between 8 to 14 hours and after at concentrations listed below must least 3 days of unrestricted diet be met or exceeded for positive (>150 g carbohydrates per day) and diagnosis. unlimited physical activity. The Fasting: 95 mg/dL patient should remain seated and 1 hour: 180 mg/dL should not smoke throughout the 2 hour: 155 mg/dL test. 3 hour: 140 mg/dL
Postpartum Fasting glucose See Gestational Diabetes Guidelines in the Normal: <100 mg/dL Prenatal Section. Abnormal: >100 mg/dL Glucose* Plasma or Plasma Equivalent Refer to Diabetes Section for guidelines. Value >21 years, male or non-pregnant Normal: Normal: female Fasting: <100 mg/dL 1. Repeat periodic screening. Notes: Random (Casual): <140 mg/dL 2. Provide counseling on nutrition, Test individuals with risk factors exercise, and risk factor reduction. listed in the Diabetes Section. Abnormal: Fasting: >100 mg/dL Abnormal: Screen all family planning Random (Casual): >140 mg/dL 1. Refer all positive results to patients for risk factors physician for follow-up. regardless of age. Note: 2. Provide counseling on nutrition, Meter must yield a plasma exercise, risk factor reduction, food Previously identified impaired equivalent value if capillary preparation and purchasing. glucose metabolism or “pre- specimen performed 3. Refer for Medical Nutrition diabetes” (fasting plasma glucose Therapy. of >100 but <126 mg/dL) OR previously identified impaired glucose tolerance (GTT 2 hr. plasma >140 but <200 mg/dL).
*For patients not diagnosed with diabetes. Waived Hemoglobin A1C Normal Reference Range See Diabetes Section and kit package 4.0-6.5 % inserts for Guidelines NOTE: Waived fingerstick Hgb A1C testing is not for the screening 6.0%- 9.0% in patients with or diagnosis of diabetes well to moderately controlled diabetes
Page 2 of 9 Kentucky Public Health Practice Reference Section: Lab July 31, 2011 Commonly Performed Lab Tests and Procedures continued (Not all-inclusive)
Laboratory Test/Procedure Findings and Directives Follow-up Guidelines Hemoglobin/Hematocrit Normal Normal Age Hgb Hct (gm/dL) (%) 1.Screen for WIC Services 1-3 days(cap) 14.5-22.5 45-61 2.For Hgb >9.0, Hct >27.0 but 1-2 wk 12.5-18.5 39-57 less than the recommended 1-6 months 10.0-13.0 29-42 normals, repeat screen in 1–3 7 mo-2 yrs 10.5-13.0 29-42 months. 2-5 yrs 11.5-13.0 34-39 3.Refer for medical evaluation 5-12 yrs 11.5-15.5 35-45 when Hgb <9.0, Hct <27.0. Male 4.Refer to appropriate age section 12-18yrs 13.0-16.0 37-49 for follow-up. Female 12-18 yrs 12.0-16.0 36-46 Male >18 yrs. 13.5-17.5 41-53 Female >18 yrs. 12.0-16.0 36-46
Pregnant Female: Abnormal Abnormal Trimester 1 < 10.9 < 32.9 Trimester 2 < 10.4 < 31.9 Trimester 3 <10.9 < 32.9 Hepatitis B Refer to Reportable Disease Desk (For Prenatal and LHD employees) Reference.
Screen individuals with the Non-reactive (Negative) Screen Household and sexual following risk factors: Repeatedly Reactive (Positive) contacts of prenatal patients who 1. Prenatal patient for initial screen positive. Test for Anti- screen, obtain HBsAg. HBc and Anti-HBs. 2. Prenatal patient with known exposure to HBsAg positive partners, obtain HBsAg, Anti-HBs and Anti- HBc Infants born to HBsAg positive 3. LHD Employee-Post- mothers should be tested 3–9 vaccine testing, obtain Anti- mos. after their third dose of HBs at 1–2 mos. post-vaccine. hepatitis vaccine. (Obtain Anti- 4. Employee Percutaneous HBs if testing post-immunization or permucosal exposure: obtain therapy.) baseline and 6 mos. (Anti-HBs) 5. Percutaneous or permucosal exposure, test source patient, obtain HBsAg.
Page 3 of 9 Kentucky Public Health Practice Reference Section: Lab July 31, 2011 Commonly Performed Lab Tests and Procedures continued (Not all-inclusive)
Laboratory Test/Procedure Findings and Directives Follow-up Guidelines HIV Testing & Counseling Non-reactive (Negative) See HIV Section for Guidance. (Male and Female) Repeatedly Reactive (Positive) POSITIVE RESULTS ARE Occupational – LHD Employee SIGNIFICANT. Percutaneous or permucosal exposure: recommended testing schedule is Baseline, 6 weeks, 3 months, 6 months, and 1 year. Lead Screening Normal: See Lead Section for guidance. (6 months to 6 years) Lead level of 0–9 µg/dL High Risk: Lead level of 10–14 µg/dL Lead Poisoning: Confirmed Lead Level of >15 µg/dL
Lipid Profile, Fasting Normal: Normal: Note: Total Cholesterol <200 mg/dL 1. Repeat screen in 5 years Screen individuals for the HDL Cholesterol(male) >60 mg/dL and at clinician’s discretion following indications: HDL Cholesterol(female) >60 mg/dL 2. Provide preventive Persons with total blood LDL Cholesterol <100 mg/dL counseling cholesterol of 200 mg/dL or Triglycerides <150 mg/dL greater Persons diagnosed with Borderline Risk: diabetes* Total Cholesterol 200-239 mg/dL Borderline/High Risk: Persons with multiple (2+) HDL Cholesterol(male) 40-59 mg/dL 1. Refer for Medical risk factors for CVD (family HDL Cholesterol(female) 50-59 mg/dL Evaluation history of premature CVD, LDL Cholesterol 130-159 mg/dL 2. Refer high-risk (>240 hypertension, hyperlipidemia, Triglycerides 150 -199 mg/dL mg/dL) for Medical Nutrition diabetes, substance abuse Therapy (MNT) at clinician’s including alcohol; cigarette High Risk: discretion. For lab values that smoking; obesity; signs and Total Cholesterol > 240 mg/dL require MNT, see Nutrition symptoms of diabetes and HDL Cholesterol(male) < 40 mg/dL Section CVD; age-men >45 and HDL Cholesterol(female) < 50 mg/dL 3. Identify with patient women >55 years; Race- LDL Cholesterol >160 mg/dL modifiable risk factors African Americans; and Triglycerides > 200 mg/dL 4. Discuss lifestyle changes physical inactivity). individualized to patient’s Obtain at least every 5 years in needs and modifiable risk adults age 21 and over factors 5. Document referral and *Note: Please refer to Diabetes return appointments Section for findings and follow-up 6. Repeat screen in 3 guidelines. months and/or at clinician’s discretion
Page 4 of 9 Kentucky Public Health Practice Reference Section: Lab July 31, 2011 Commonly Performed Lab Tests and Procedures continued (Not all-inclusive)
Laboratory Test/Procedure Findings and Directives Follow-up Guidelines Metabolic (Newborn) Screening Findings and Directives for all (Birth to 6 months of age) Metabolic Screening Disorders MS/MS tests by Category Normal No further action necessary Amino Acid Disorders: Argininosuccinate Acidemia, Citrullinemia, Abnormal Laboratory will make immediate Homocystinuria, Maple Syrup Urine Disease, referral to university specialist. Phenylketonuria, Tyrosinemia, Argininemia, NBS follow-up staff will notify Hyperphenylalaninemia,Hypermethioninemia PCP of referral and provide , educational materials for PCP Nonketotic Hyperglycinemia and parents.
Fatty Acid Disorders: Unsatisfactory, and Normal Results that apply to all disorders Carnitine uptake defect, Long-chain 3- but <24 Hours of Age are in the Newborn Screening panel. hydroxyacyl-CoA dehydrogenase deficiency results that apply to all Follow-up guidelines will be the (LCHAD), Medium-chain acyl-CoA disorders in the Newborn same for all disorders with these dehydrogenase deficiency (MCAD), Short- Screening panel. Follow-up results. chain acyl-CoA dehydrogenase deficiency Guidelines will be the same (SCAD), Trifunctional protein deficiency, for all disorders with these Very long-chain acyl-CoA dehydrogenase results. deficiency (VLCAD) ), Carnitine Rescreen as directed in report acylcarnitine translocase deficiency,Carnitine Unsatisfactory palmitoyl transferase deficiency,Glutaric academia type II. <24 hours of age Rescreen
Organic Acid Disorders: 3-methylcrotonyl CoA-Carboxylase Deficiency, Beta-ketothiolase, Glutaric acidemia type 1, Isovaleric acidemia, 3- Transfusion Transfusion Management: hydroxy 3-methylglutaric aciduria, Rescreen for all disorders 72 hrs Methylmalonic acidemia, methylmalonic after transfusion. acidemia mutase deficiency, Propionic Acidemia, Multiple carboxylase deficiency,2- 90 days after transfusion, Methyl-3-Hydroxybutyric aciduria, 3- rescreen for any disorder that Methylglutaconic acidura, Isobutyryl-CoA relies on red blood cell analysis dehydrogenase deficiency, Malonic acidemia, such as hemoglobinopathies, Ethylmalonic encephalopathy, 2- galactosemia, and biotinidase. Methylbutyryl-CoA dehydrogenase deficiency. See Specific Test Reports- Endocrine Disorders Follow-up as clinically indicated Congenital Adrenal Hyperplasia, Congenital Hypothyroidism
Hemoglobinopathies HbSS HbSC HBSβ0 HbSβ+
Other Disorders Utilizing Red Blood Cell Analysis
Page 5 of 9 Kentucky Public Health Practice Reference Section: Lab July 31, 2011 Biotinidase Deficiency Galactosemia
Other Disorders Cystic Fibrosis
Submit filter paper specimen to the Division of Laboratory Services, make sure filter paper is “in-date”, not expired. Allow specimen to dry for at least 3 hours before mailing and mail to Lab within 24 hours of obtaining.
Page 6 of 9 Kentucky Public Health Practice Reference Section: Lab July 31, 2011 Ova and Parasites Presence (positive) or Recommend medical evaluation Note: for acute or chronic condition. Symptoms may include: abdominal discomfort – cramps with diarrhea containing Absence (none found, blood or mucus, chronic diarrhea. negative) Transmitted fecal-oral route. Pinworm Prep Presence (positive) or Recommend medical evaluation Note: for acute or chronic condition. Symptoms include: abdominal discomfort, with pruritus ani, especially at night. Absence (none found, Transmitted fecal-oral route. negative) Pregnancy Test, urine Negative or Positive See pregnancy test guidelines in Family Planning Section. Rubella Negative : Consistent with NO Negative immune status: immunity 1. Determine pregnancy status by history, date of LMP, and/or pregnancy test if necessary. 2. Offer one MMR vaccine to persons regardless of age who have never been immunized against MMR. 3. If patient is under age 21 and has never had MMR vaccine, can offer first and booster doses of MMR. 4. Counsel to avoid pregnancy for at least three Equivocal, Borderline or months. Uncertain Immune status Equivocal immune status: An equivocal value on two specimens 14 days apart may indicate a vaccine booster is needed. See management above Positive: Consistent with (negative). Immunity Positive immune status: 1. Inform patient of evidence of protection against rubella 2. Inquire about history of vaccination against measles and mumps 3. Manage as above if no evidence of protection against measles and mumps
Page 7 of 9 Kentucky Public Health Practice Reference Section: Lab July 31, 2011 Commonly Performed Lab Tests and Procedures continued (Not all-inclusive)
Laboratory Test/Procedure Findings and Directives Follow-up Guidelines Urinalysis Positive or Negative Recommend medical evaluation for acute or chronic conditions. Positive dipstick for Leukocytes, Nitrites, Glucose, Ketone bodies, Protein or Blood. VDRL Non-reactive
Reactive: Confirmatory See STD Section for Guidance. testing performed at the State Lab, including EIA, TPPA, FTA testing. See Specific Report.
Weakly reactive: See STD Section for Guidance. Confirmatory testing performed at the State Lab, including EIA, TPPA, FTA testing. See Specific Report. Wet Mount Normal
Abnormal Refer to LHD Lab procedure manual and White Blood Cells STD section Bacterial Overgrowth Clue Cells Trichomonas Yeast/budding yeast
Page 8 of 9 Kentucky Public Health Practice Reference Section: Lab July 31, 2011 Shipping Laboratory Specimens to Division of Laboratory Services (DLS)
Shipping containers and color-coded postage shipping labels are provided to the health department from DLS for the purpose of shipping specimens. - For definitions of the color-coded postage shipping labels and listing of possible shipping methods, see the Attachments Section of Volume I of the Administrative Reference.
Packaging and Shipping of Infectious Substances
Department of Transportation (DOT) 49 CFR (http://www.phmsa.dot.gov/regulations/), Domestic Mail Manual (DMM) (http://pe.usps.gov/text/dmm300/601.htm#wp1064962/), and International Air Transport Association (IATA) guidelines must be followed in determining if a specimen for shipping is classified as infectious substance category A or biological substance category B. Employees responsible for infectious substance packaging and shipping (either category A or B) must be trained and certified by their employer within 90 days of employment. The training guidelines are found in the DOT regulations. Training for packaging and shipping infectious substances must occur every 3 years if shipping solely by USPS; Every 2 years if shipping by UPS or FedEx.
Page 9 of 9 Kentucky Public Health Practice Reference Section: Lab July 31, 2011