Housed in Silwood Park Library
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

BSBI SCOTTISH KEWSIETTER Lumber 2 Summer 1980
B.S.B. I. SCOTTISH NEWSLETTER BSBI SCOTTISH KEWSIETTER lumber 2 Summer 1980 COOTEOTS Editorial 2 Woodsia ilvensis in the Itoffat area 2 Ulex gallii in the far north of Scotland - E.R.Bullard 5 Cirsium wankelii in Argyll, v.c.98 - A.G*Kenneth 6 Discovery of Schoenus ferrugineous as a native British plant - R.A.H. Smith 7 Rosa arvensis in Scotland - O.M. Stewart 7 The year of the Dandelion - G.H. Ballantyne 8 •The Flora of Kintyre1. Review - P. Macpherson 9 Liaison between the BSBI and Nature Conservancy Council 10 HCC Assistant Regional Officers 10 Calamagrostis - 0«M. Stewart 13 Spiraea salicifolia group - A.J, Silverside 13 % Chairman's Letter 14 Flora of Uig (Lewis) 15 Cover Illustration - Woodsia ilvensis by Olga M. Stewart 1 EDITORIAL staff, who is the author of a recent report on the subjecto * We hope that this, the second number of the newsletter, meets with the approval of our readers, but its success The report is divided into four parts - Geographical can only be judged by our receiving your comments and distribution? Ecological aspects? Past and Present Status constructive criticism, so please let us know what you in the Moffat Hills? and Conservation in Britain. The think of our efforts® world, European and British distribution is discussed in Our cover illustration, for which we are once again general terms, with appropriate maps. These show W. indebted to Mrs Olga Stewart, has been chosen as an ilvensis to be a fern of the i'orth Temperate zone, con fined to the Arctic and mountainous regions, extending as accompaniment to the item on Woodsia ilvensis. -

2013 Draft Mazama Pocket Gopher Status Update and Recovery Plan
DRAFT Mazama Pocket Gopher Status Update and Recovery Plan Derek W. Stinson Washington Department of Fish and Wildlife Wildlife Program 600 Capitol Way N Olympia, Washington January 2013 In 1990, the Washington Wildlife Commission adopted procedures for listing and de-listing species as endangered, threatened, or sensitive and for writing recovery and management plans for listed species (WAC 232-12-297, Appendix A). The procedures, developed by a group of citizens, interest groups, and state and federal agencies, require preparation of recovery plans for species listed as threatened or endangered. Recovery, as defined by the U.S. Fish and Wildlife Service, is the process by which the decline of an endangered or threatened species is arrested or reversed, and threats to its survival are neutralized, so that its long-term survival in nature can be ensured. This is the Draft Washington State Status Update and Recovery Plan for the Mazama Pocket Gopher. It summarizes what is known of the historical and current distribution and abundance of the Mazama pocket gopher in Washington and describes factors affecting known populations and its habitat. It prescribes strategies to recover the species, such as protecting populations and existing habitat, evaluating and restoring habitat, and initiating research and cooperative programs. Target population objectives and other criteria for down-listing to state Sensitive are identified. As part of the State’s listing and recovery procedures, the draft recovery plan is available for a 90-day public comment period. Please submit written comments on this report by 19 April 2013 via e-mail to: [email protected], or by mail to: Endangered Species Section Washington Department of Fish and Wildlife 600 Capitol Way North Olympia, WA 98501-1091 This report should be cited as: Stinson, D. -

Area 3 Habitat Assessments and Pollinator Surveys October
Inspiring change for Important Invertebrate Areas in the UK Area 3 habitat assessments and pollinator surveys 11th September 2014 Susan Thompson - Grants & Trusts Officer October 2016 (Updated April 2017) Jamie Robins Saving the small things that run the planet Executive Summary Buglife Services were commissioned by Kier Group Ltd in July 2016 to undertake a series of baseline habitat surveys within the Area 3 network, to identify sites where improvements for pollinators could be implemented. These results were then used to identify ten sites with the highest potential and deliverability, using a ranking exercise, in addition to a Kier owned depot. Buglife Services undertook more detailed habitat assessments and pollinator surveys during brief site visits in August 2016. Of the 11 sites surveyed, a total of 104 pollinator species were recorded. This comprised 18 Lepidoptera (butterflies and moths), 33 Hoverflies, 18 other Diptera (true flies), 14 bees, 6 other hymenoptera and 11 other minor pollinators (e.g. true bugs and beetles). A further 38 incidental (non-pollinator) species were also recorded. These results and habitat assessments were used to provide management suggestions to enhance the value of these sites for pollinators. Where possible suggestions aimed to benefit key species recorded during site surveys, such as the Chalk hill blue butterfly (Polyommatus coridon), the Section 41 priority species Straw belle moth (Aspitates gilvaria) and the scarce (Nb) wasp Microdynerus exilis. Finally, recommendations for future survey and monitoring were provided, aiming to inform Kier of the progression of habitat improvements for pollinators and advise future management. Introduction There has been a growing interest in managing road verges and other transport features more sensitively for biodiversity. -

Oberholzeria (Fabaceae Subfam. Faboideae), a New Monotypic Legume Genus from Namibia
RESEARCH ARTICLE Oberholzeria (Fabaceae subfam. Faboideae), a New Monotypic Legume Genus from Namibia Wessel Swanepoel1,2*, M. Marianne le Roux3¤, Martin F. Wojciechowski4, Abraham E. van Wyk2 1 Independent Researcher, Windhoek, Namibia, 2 H. G. W. J. Schweickerdt Herbarium, Department of Plant Science, University of Pretoria, Pretoria, South Africa, 3 Department of Botany and Plant Biotechnology, University of Johannesburg, Johannesburg, South Africa, 4 School of Life Sciences, Arizona a11111 State University, Tempe, Arizona, United States of America ¤ Current address: South African National Biodiversity Institute, Pretoria, South Africa * [email protected] Abstract OPEN ACCESS Oberholzeria etendekaensis, a succulent biennial or short-lived perennial shrublet is de- Citation: Swanepoel W, le Roux MM, Wojciechowski scribed as a new species, and a new monotypic genus. Discovered in 2012, it is a rare spe- MF, van Wyk AE (2015) Oberholzeria (Fabaceae subfam. Faboideae), a New Monotypic Legume cies known only from a single locality in the Kaokoveld Centre of Plant Endemism, north- Genus from Namibia. PLoS ONE 10(3): e0122080. western Namibia. Phylogenetic analyses of molecular sequence data from the plastid matK doi:10.1371/journal.pone.0122080 gene resolves Oberholzeria as the sister group to the Genisteae clade while data from the Academic Editor: Maharaj K Pandit, University of nuclear rDNA ITS region showed that it is sister to a clade comprising both the Crotalarieae Delhi, INDIA and Genisteae clades. Morphological characters diagnostic of the new genus include: 1) Received: October 3, 2014 succulent stems with woody remains; 2) pinnately trifoliolate, fleshy leaves; 3) monadel- Accepted: February 2, 2015 phous stamens in a sheath that is fused above; 4) dimorphic anthers with five long, basifixed anthers alternating with five short, dorsifixed anthers, and 5) pendent, membranous, one- Published: March 27, 2015 seeded, laterally flattened, slightly inflated but indehiscent fruits. -

Die Raupenfliegen (Diptera: Tachinidae) Mitteleuropas: Bestimmungstabellen Und Angaben Zur Verbreitung Und Ökologie Der Einzelnen Arten
5 download Biodiversity Heritage Library, http://www.biodiversitylibrary.org/ Stuttgarter Beiträge zur Naturkunde Serie A (Biologie) Herausgeber: 4fr für Naturkunde, RosensteinV 70 19 l; , Staatliches Museum 1, D- r Stuttgart Stuttgarter Beitr. Naturk. Ser. A Nr. 506 170 S ,4. 9. 19.94 Professor Dr. Bernhard Ziegler zum 65. Geburtstag Die Raupenfliegen (Diptera: Tachinidae) Mitteleuropas: Bestimmungstabellen und Angaben zur Verbreitung und Ökologie der einzelnen Arten The Tachinids (Diptera: Tachinidae) of Central Europe: Identification Keys for the Species and Data on Distribution and Ecology Von Hans-Peter Tschorsnig und Benno Herting, Stuttgart Mit 291 Abbildungen Summary Keys are given for all central and northern European species of Tachinidae (Diptera). The most important data on distribution and ecology (mainly habitat, phenology, and host-range) are listed for the central European species. Zuammenfassung Es werden Bestimmungsschlüssel für alle in Mittel- und Nordeuropa vorkommenden Arten der Tachinidae (Diptera) gegeben. Für jede mitteleuropäische Art werden die wichtigsten Kenndaten zur Verbreitung und Ökologie (vor allem Habitat, Flugzeit und Wirtskreis) aufge- listet. Inhalt 1. Einleitung 2 2. Beg'iff.c erklärungen 4 2.1. Allgemeines 4 2.2. Kopf 5 2.3. Thorax 7 2.4. Flügel 8 2.5. Beine 9 2.6. Abdomen 10 2.7. Bereifung 11 2.8. Färbung . 11 download Biodiversity Heritage Library, http://www.biodiversitylibrary.org/ 2 STUTTGARTER BEITRÄGE ZUR NATURKUNDE Ser. A, Nr. 506 2.9. Körpergröße 11 2.10. Abkürzungen 11 3. Schlüssel für die Gattungen 12 4. Schlüssel für die Arten . 42 4.1. Subfamilie Exoristinae 42 4.2. Subfamilie Tachininae 63 4.3. Subfamilie Dexiinae 79 4.4. -

Lepidopterous Fauna Lancashire and Cheshire
LANCASHIRE AND CHESHIRE LEPIDOPTERA, THE LEPIDOPTEROUS FAUNA OF LANCASHIRE AND CHESHIRE COMPILED BY WM. MANSBRIDGE, F.E.S., Hon. Sec. La11c:1 shire and Cheshire Entomological Society. BEING A NEW EDITION OF Dr. ELLIS'S LIST brought up to date with the a~s istance of the Lepidoptcrists whose names nppcnr below. Ark le, J., Chester A. Baxter, T., Min-y-don, St. Annes-on-Sea T.B. Bell, Dr. Wm., J.P., Rutland House, New Brighton W.B. Boyd, A. W., M.A., F.E.S., The Alton, Altrincham ... A.W.B Brockholes, J. F. The late J.F.B. Capper, S. J. The late .. S.J.C. Chappell, Jos. The late .. J C. Collins, Joseph, The University Museum, Oxford J. Coll. Cooke, N. The late N.C. Corbett, H. H., Doncaster H.H.C. Cotton, J., M.R.C.S., etc., Simonswood, Prescot Rd., St. Helens ... ]. Cot. Crabtree, B. H., F. E.S., Cringle Lodge, Leve nshulme, Manchester ... B.H.C. Day, G. 0 ., F.E.S. late of Knutsforcl ... D. Wolley-Dod, F. H, Edge, near Malpas F.H.W.D. Ellis, John W ., M.B. (Vic), F.E.S., etc., 18, Rodney Street, Liverpool J.W.E. Forsythe, Claude F., The County Asylum, Lancaster C.H F. Frewin, Colonel, Tarvin Sands ... F. Greening, Noah, The late N.G. Gregson, Chas. S., The late C.S.G. Gregson, W., The late ... W.G. Harrison, Albert, F.E.S., The lalt1 A.H. 2 LANCASHIRE AND CHESHIRE LEPIDOPTERA. LANCASHIRE AND CHESHIRE LEPIDOPTERA. 3 Harrison, W. W.H. Higgins, Rev: H. -

Bilimsel Araştırma Projesi (8.011Mb)
1 T.C. GAZİOSMANPAŞA ÜNİVERSİTESİ Bilimsel Araştırma Projeleri Komisyonu Sonuç Raporu Proje No: 2008/26 Projenin Başlığı AMASYA, SİVAS VE TOKAT İLLERİNİN KELKİT HAVZASINDAKİ FARKLI BÖCEK TAKIMLARINDA BULUNAN TACHINIDAE (DIPTERA) TÜRLERİ ÜZERİNDE ÇALIŞMALAR Proje Yöneticisi Prof.Dr. Kenan KARA Bitki Koruma Anabilim Dalı Araştırmacı Turgut ATAY Bitki Koruma Anabilim Dalı (Kasım / 2011) 2 T.C. GAZİOSMANPAŞA ÜNİVERSİTESİ Bilimsel Araştırma Projeleri Komisyonu Sonuç Raporu Proje No: 2008/26 Projenin Başlığı AMASYA, SİVAS VE TOKAT İLLERİNİN KELKİT HAVZASINDAKİ FARKLI BÖCEK TAKIMLARINDA BULUNAN TACHINIDAE (DIPTERA) TÜRLERİ ÜZERİNDE ÇALIŞMALAR Proje Yöneticisi Prof.Dr. Kenan KARA Bitki Koruma Anabilim Dalı Araştırmacı Turgut ATAY Bitki Koruma Anabilim Dalı (Kasım / 2011) ÖZET* 3 AMASYA, SİVAS VE TOKAT İLLERİNİN KELKİT HAVZASINDAKİ FARKLI BÖCEK TAKIMLARINDA BULUNAN TACHINIDAE (DIPTERA) TÜRLERİ ÜZERİNDE ÇALIŞMALAR Yapılan bu çalışma ile Amasya, Sivas ve Tokat illerinin Kelkit havzasına ait kısımlarında bulunan ve farklı böcek takımlarında parazitoit olarak yaşayan Tachinidae (Diptera) türleri, bunların tanımları ve yayılışlarının ortaya konulması amaçlanmıştır. Bunun için farklı böcek takımlarına ait türler laboratuvarda kültüre alınarak parazitoit olarak yaşayan Tachinidae türleri elde edilmiştir. Kültüre alınan Lepidoptera takımına ait türler içerisinden, Euproctis chrysorrhoea (L.), Lymantria dispar (L.), Malacosoma neustrium (L.), Smyra dentinosa Freyer, Thaumetopoea solitaria Freyer, Thaumetopoea sp. ve Vanessa sp.,'den parazitoit elde edilmiş, -

Species List Millers Pond 2019
Sheet1 Miller's Pond Local Nature Reserve: Bioblitz 6 to 7 September 2019. Checklist by P A Budd Taxon Species Grid Square Date Recorder Notes Acanthosoma haemorrhoidale Hawthorn Shieldbug SU451110 07/09/19 Summer Harrod Acer campestre Field Maple SU451109 07/09/19 Lindsay McCulloch Acer pseudoplatanus Sycamore SU451110 07/09/19 Natalie Hands Acer pseudoplatanus Sycamore SU452110 07/09/19 Lindsay McCulloch Achillea millefolium Yarrow SU451110 07/09/19 Philip Budd Achillea millefolium Yarrow SU452110 07/09/19 Lindsay McCulloch Aelia acuminata Bishop's Mitre Shieldbug SU452109 07/09/19 Richard Dickson Sweeping Aeshna cyanea Southern Hawker SU450110 07/09/19 Terry Crow 3 seen Aeshna mixta Migrant Hawker SU450110 07/09/19 Terry Crow Agelastica alni Alder Leaf Beetle SU452109 07/09/19 Richard Dickson On nettle Anas platyrhynchos Mallard SU450110 07/09/19 Andy Bones Anas platyrhynchos Mallard SU450109 07/09/19 Dorman family 8 seen Anax imperator Emperor Dragonfly SU450109 07/09/19 Dorman family Anax imperator Emperor Dragonfly SU450110 07/09/19 Dorman family Anax imperator Emperor Dragonfly SU451110 07/09/19 Philip Budd 2 seen Andricus quercuscalcis Knopper Gall SU451110 07/09/19 Steve Groom Galls on oak Anyphaena accentuata a spider SU451110 07/09/19 Carly Harrod Araneus diadematus Garden Spider SU450109 07/09/19 Steve Groom Araneus diadematus Garden Spider SU450109 07/09/19 Terry Crow Araneus diadematus Garden Spider SU453110 07/09/19 Terry Crow Araneus diadematus Garden Spider SU450110 07/09/19 Daniel Bocock 2 seen Araneus diadematus Garden Spider SU450110 07/09/19 Dorman family 2 seen Araneus diadematus Garden Spider SU451110 06/09/19 Philip Budd Immature Araneus marmoreus var. -

Micro Moths on Great Cumbrae Island (Vc100)
The Glasgow Naturalist (online 2017) Volume 26, xx-xx Micro moths on Great Cumbrae Island (vc100) P. G. Moore 32 Marine Parade, Millport, Isle of Cumbrae KA28 0EF E-mail: [email protected] ABSTRACT Forsythia sp. Behind the office is a large mature Few previous records exist for miCro-moths from black mulberry tree (Morus nigra) and to one side is vC100. Data are presented from the first year-round a tall privet hedge (Ligustrum ovalifolium). To the moth-trapping exerCise accomplished on Great rear of my property is a wooded escarpment with Cumbrae Island; one of the least studied of the old-growth ash (Fraxinus excelsior) frequently ivy- Clyde Isles (vC100). Data from a Skinner-type light- Covered (Hedera helix), sycamore (Acer trap, supplemented by Collection of leaf mines from pseudoplatanus) and rowan (Sorbus aucuparia), local trees, revealed the presence of 71 species of with an undergrowth of hawthorn (Crataegus miCro moths, representing 20 new records for the monogyna), wild garliC (Allium ursinum), nettle vice-County. (Urtica dioica), bracken (Pteridium aquilinum) and bramble (Rubus fructicosus). Rhind (1988) detailed INTRODUCTION the vasCular plants found on Great Cumbrae Island The extensive nineteenth-century list of between 1985 and 1987 and delineated the history Lepidoptera in the 1901 handbook on the natural of the island's botanical investigations. Leaves of history of Glasgow and the West of SCotland issued brambles in my garden, beech trees (Fagus for the Glasgow meeting of the British AssoCiation sylvatica) and hazel (Corylus avellana) at other for the Advancement of SCience (Elliot et al., 1901) locations on the island (respectively Craiglea Wood inCluded few Cumbrae records. -
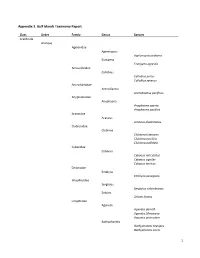
1 Appendix 3. Gulf Islands Taxonomy Report
Appendix 3. Gulf Islands Taxonomy Report Class Order Family Genus Species Arachnida Araneae Agelenidae Agelenopsis Agelenopsis utahana Eratigena Eratigena agrestis Amaurobiidae Callobius Callobius pictus Callobius severus Antrodiaetidae Antrodiaetus Antrodiaetus pacificus Anyphaenidae Anyphaena Anyphaena aperta Anyphaena pacifica Araneidae Araneus Araneus diadematus Clubionidae Clubiona Clubiona lutescens Clubiona pacifica Clubiona pallidula Cybaeidae Cybaeus Cybaeus reticulatus Cybaeus signifer Cybaeus tetricus Dictynidae Emblyna Emblyna peragrata Gnaphosidae Sergiolus Sergiolus columbianus Zelotes Zelotes fratris Linyphiidae Agyneta Agyneta darrelli Agyneta fillmorana Agyneta protrudens Bathyphantes Bathyphantes brevipes Bathyphantes keeni 1 Centromerita Centromerita bicolor Ceratinops Ceratinops latus Entelecara Entelecara acuminata Erigone Erigone aletris Erigone arctica Erigone cristatopalpus Frederickus Frederickus coylei Grammonota Grammonota kincaidi Linyphantes Linyphantes nehalem Linyphantes nigrescens Linyphantes pacificus Linyphantes pualla Linyphantes victoria Mermessus Mermessus trilobatus Microlinyphia Microlinyphia dana Neriene Neriene digna Neriene litigiosa Oedothorax Oedothorax alascensis Pityohyphantes Pityohyphantes alticeps Pocadicnemis Pocadicnemis pumila Poeciloneta Poeciloneta fructuosa Saaristoa Saaristoa sammamish Scotinotylus Scotinotylus sp. 5GAB Semljicola Semljicola sp. 1GAB Sisicottus Spirembolus Spirembolus abnormis Spirembolus mundus Tachygyna Tachygyna ursina Tachygyna vancouverana Tapinocyba Tapinocyba -

Additions, Deletions and Corrections to An
Bulletin of the Irish Biogeographical Society No. 36 (2012) ADDITIONS, DELETIONS AND CORRECTIONS TO AN ANNOTATED CHECKLIST OF THE IRISH BUTTERFLIES AND MOTHS (LEPIDOPTERA) WITH A CONCISE CHECKLIST OF IRISH SPECIES AND ELACHISTA BIATOMELLA (STAINTON, 1848) NEW TO IRELAND K. G. M. Bond1 and J. P. O’Connor2 1Department of Zoology and Animal Ecology, School of BEES, University College Cork, Distillery Fields, North Mall, Cork, Ireland. e-mail: <[email protected]> 2Emeritus Entomologist, National Museum of Ireland, Kildare Street, Dublin 2, Ireland. Abstract Additions, deletions and corrections are made to the Irish checklist of butterflies and moths (Lepidoptera). Elachista biatomella (Stainton, 1848) is added to the Irish list. The total number of confirmed Irish species of Lepidoptera now stands at 1480. Key words: Lepidoptera, additions, deletions, corrections, Irish list, Elachista biatomella Introduction Bond, Nash and O’Connor (2006) provided a checklist of the Irish Lepidoptera. Since its publication, many new discoveries have been made and are reported here. In addition, several deletions have been made. A concise and updated checklist is provided. The following abbreviations are used in the text: BM(NH) – The Natural History Museum, London; NMINH – National Museum of Ireland, Natural History, Dublin. The total number of confirmed Irish species now stands at 1480, an addition of 68 since Bond et al. (2006). Taxonomic arrangement As a result of recent systematic research, it has been necessary to replace the arrangement familiar to British and Irish Lepidopterists by the Fauna Europaea [FE] system used by Karsholt 60 Bulletin of the Irish Biogeographical Society No. 36 (2012) and Razowski, which is widely used in continental Europe. -

Monitoring Report Spring/Summer 2015 Contents
Wimbledon and Putney Commons Monitoring Report Spring/Summer 2015 Contents CONTEXT 1 A. SYSTEMATIC RECORDING 3 METHODS 3 OUTCOMES 6 REFLECTIONS AND RECOMMENDATIONS 18 B. BIOBLITZ 19 REFLECTIONS AND LESSONS LEARNT 21 C. REFERENCES 22 LIST OF FIGURES Figure 1 Location of The Plain on Wimbledon and Putney Commons 2 Figure 2 Experimental Reptile Refuge near the Junction of Centre Path and Somerset Ride 5 Figure 3 Contrasting Cut and Uncut Areas in the Conservation Zone of The Plain, Spring 2015 6/7 Figure 4 Notable Plant Species Recorded on The Plain, Summer 2015 8 Figure 5 Meadow Brown and white Admiral Butterflies 14 Figure 6 Hairy Dragonfly and Willow Emerald Damselfly 14 Figure 7 The BioBlitz Route 15 Figure 8 Vestal and European Corn-borer moths 16 LIST OF TABLES Table 1 Mowing Dates for the Conservation Area of The Plain 3 Table 2 Dates for General Observational Records of The Plain, 2015 10 Table 3 Birds of The Plain, Spring - Summer 2015 11 Table 4 Summary of Insect Recording in 2015 12/13 Table 5 Rare Beetles Living in the Vicinity of The Plain 15 LIST OF APPENDICES A1 The Wildlife and Conservation Forum and Volunteer Recorders 23 A2 Sward Height Data Spring 2015 24 A3 Floral Records for The Plain : Wimbledon and Putney Commons 2015 26 A4 The Plain Spring and Summer 2015 – John Weir’s General Reports 30 A5 a Birds on The Plain March to September 2015; 41 B Birds on The Plain - summary of frequencies 42 A6 ai Butterflies on The Plain (DW) 43 aii Butterfly long-term transect including The Plain (SR) 44 aiii New woodland butterfly transect