Key to Review Worksheet for Exam 2 Chem 103, Winter 2006
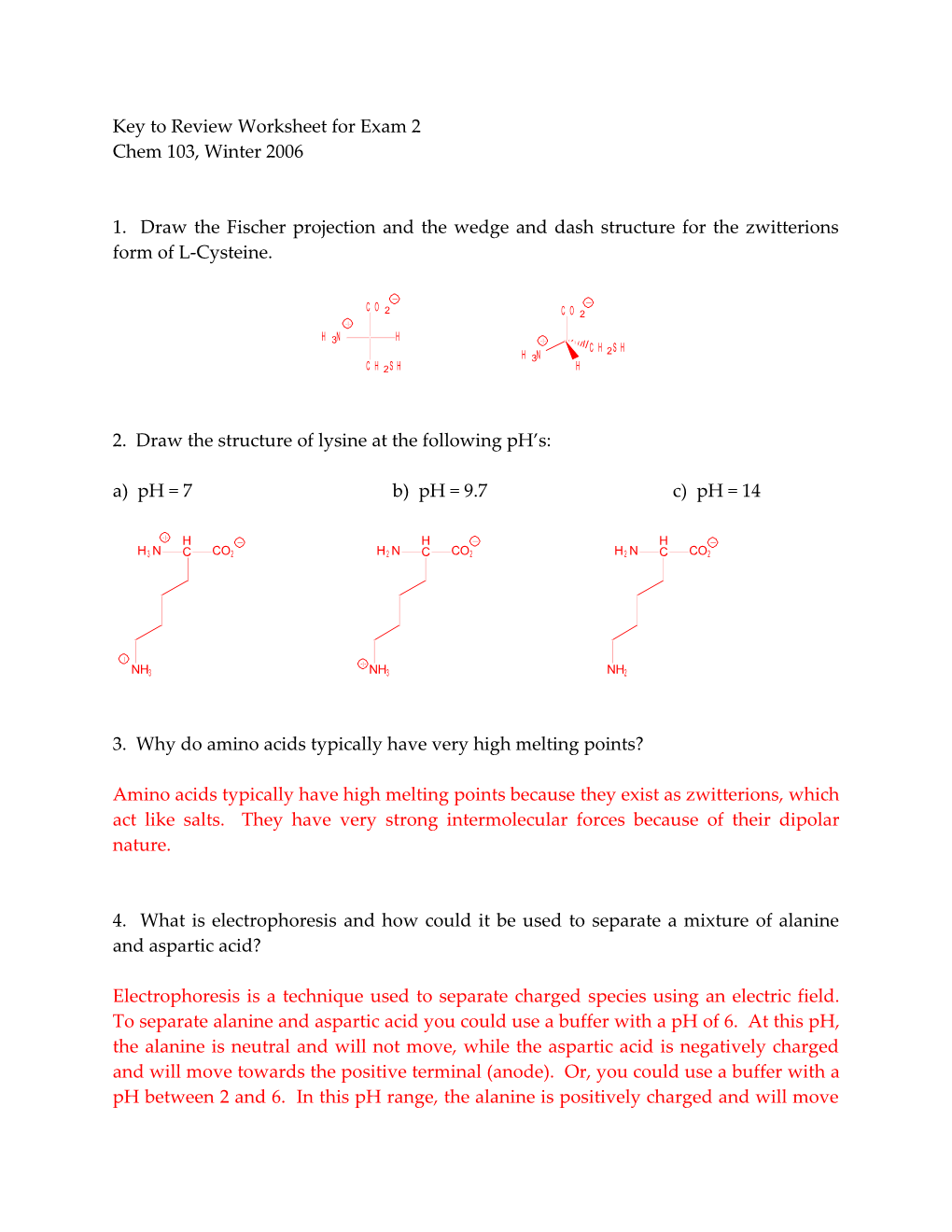
1. Draw the Fischer projection and the wedge and dash structure for the zwitterions form of L-Cysteine.
C O 2 C O 2
H 3N H C H 2 S H H 3N C H 2 S H H
2. Draw the structure of lysine at the following pH’s: a) pH = 7 b) pH = 9.7 c) pH = 14
H H H H 3 N C CO2 H 2 N C CO2 H 2 N C CO2
NH3 NH3 NH2
3. Why do amino acids typically have very high melting points?
Amino acids typically have high melting points because they exist as zwitterions, which act like salts. They have very strong intermolecular forces because of their dipolar nature.
4. What is electrophoresis and how could it be used to separate a mixture of alanine and aspartic acid?
Electrophoresis is a technique used to separate charged species using an electric field. To separate alanine and aspartic acid you could use a buffer with a pH of 6. At this pH, the alanine is neutral and will not move, while the aspartic acid is negatively charged and will move towards the positive terminal (anode). Or, you could use a buffer with a pH between 2 and 6. In this pH range, the alanine is positively charged and will move towards the negative electrode (cathode), while the aspartic acid will be neutral and will not move.
5. Draw the structure of Ser-Phe-Gly. Label the C-terminus and the N-terminus.
O O O H H H3N C C N C C N C C O H H H2 N-term. CH2 CH2 C-term.
OH
6. Di-peptides are more stable to acid hydrolysis than disaccharides. What makes the peptide bond so particularly stable and strong? Use drawings to illustrate your answer.
Di-peptides contain amide bonds, while disaccharides contain ether bonds (they are acetals). The lone-pair of electrons on the amide nitrogen is delocalized through the carbonyl pi system, while the lone-pairs of electrons on the ether oxygen are localized on that oxygen. This is because nitrogen is less electronegative than oxygen, and so can better stabilize a positive charge. More electron delocalization = more stable.
O O
NH2 NH2
7. What do each of the following structural levels in proteins represent? a) primary
The primary structure represents the amino acid sequence of the protein. b) quaternary
The quaternary structure represents the joining of more than one polypeptide subunit to form a functional protein. 8. How is a polypeptide held together in an -helix?
An -helix is held together by hydrogen bonding between the backbone amide hydrogen in one loop and the backbone carbonyl oxygen on an adjacent loop.
9. What amino acids are most prevalent in -pleated sheets?
Amino acids with small side-chains, like glycine, alanine and serine are most prevalent in -pleated sheets.
10. What type of cross-linking mainly holds the helices together in -keratins?
The helices in the fibrils of -keratins are mainly held together by disulfide bonds between cysteines, which are abundant in these fibrous proteins.
11. What are salt-bridges?
A salt-bridge is an ionic bond between ionized side-chains of acidic and basic amino acids. Salt-bridges are involved in cross-linking in tertiary and quaternary structure.
12. How does isopropyl alcohol (rubbing alcohol) act as a disinfectant?
The alcohol forms hydrogen bonds with the amino acids of proteins, disrupting their secondary, tertiary and quaternary structures. Isopropyl alcohol disinfects by passing through the cell walls of bacteria, where it denatures their proteins.
13. How does a catalyst speed up a chemical reaction?
A catalyst speeds up a chemical reaction by providing an alternate pathway for the reaction that has a lower activation energy than the uncatalyzed reaction.
14. Assign a name for an enzyme that would catalyze each of the following reactions: a) oxidizes serine b) hydrolyzes lactose
Serine oxidase Lactase
15. How does the induced-fit model explain how hexokinase can recognize both glucose and fructose?
According to the induced-fit model, as the substrate binds the enzyme, they each adjust their structure to better fit each other. The substrate takes on a conformation that is similar to the transition state. So, the enzyme may recognize more than one substrate if the transition states for the substrates are similar.
16. What are isoenzymes, and how are they used to diagnose specific tissue damage?
Isoenzymes are different forms of an enzyme that catalyze the same reaction, but in different parts of the organism. They are quaternary proteins with different combinations of subunits. Levels of isoenzymes from specific tissues can be measured in the blood to determine if that tissue has been damaged. (When the cells die, their contents, including enzymes, are released.)
17. If an enzyme has a maximum activity at 40C, would the activity be most reduced at 30C or at 50C? Explain.
At lower temperatures, reaction rates are slowed because there is not enough energy available to surpass the activation energy. At higher temperatures, the enzyme is denatured and completely loses activity. Thus, activity falls off more quickly above the ideal temperature than below it. The activity would be more reduced at 50C.
18. Draw a plot of reaction rate versus substrate concentration for a typical enzyme catalyzed reaction. Explain the shape of the curve of your plot. Reaction Rate
Substrate Concentration
The rate of reaction increases with increasing substrate concentration until all the enzymes are saturated, then the rate becomes constant.
19. Why does increasing the substrate concentration reverse the inhibition by a competitive inhibitor?
A competitive inhibitor works by binding to the active site of the enzyme, so that the substrate can’t bind. However, the inhibitor comes on and off the enzyme, and when it’s off, the substrate can bind. So, if the substrate concentration is high relative to the inhibitor concentration, then substrate can out-compete the inhibitor for binding.
20. What is an irreversible inhibitor, and why are they so toxic?
An irreversible inhibitor is one that doesn’t come off the enzyme once it binds. It usually forms a covalent bond with an amino acid side-chain in the active site. They are so toxic because they completely destroy enzyme function, making it impossible to compensate by an increase in substrate concentration.
21. Antibiotics are irreversible inhibitors. Why are they so toxic to bacteria and not to us?
Antibiotics are toxic to bacteria and not to us because they are specific inhibitors for bacterial enzymes, and are not recognized by our own enzymes.
22. Make a drawing to show how a noncompetitive inhibitor works. 23. What are zymogens, and how are they useful in digestion?
Zymogens are enzymes that are not produced in their active form. They are activated by removal of small peptide sections. They are useful in digestion because they can be stored in an inactive form, so they don’t injure surrounding tissue, and then be released and activated rapidly when needed.
24. How does feedback control work? Make a drawing to illustrate your answer.
Feedback control is a mechanism by which the production of a product is regulated by its local concentration. The product is a noncompetitive inhibitor for the first enzyme in a series of enzymes for a reaction. So, when lots of product is around, its production is reduced. 25. Is a coenzyme the same thing as a cofactor? Explain.
A coenzyme is a type of cofactor. A cofactor is a small molecule or metal ion that is required for an enzyme to be active. A coenzyme is a small organic molecule cofactor, such as a vitamin.
26. What are two common functions of metal ions as cofactors?
Metal ions often are involved in electron transfer (redox) reactions in the active site. They also can activate the substrate by interacting with electron-rich atoms on the amino acid side-chains. They stabilize the transition state, and so lower the activation energy for the catalyzed reaction.
27. What is a vitamin?
A vitamin is an organic molecule that is essential, but cannot be biosynthesized, so must be consumed in the diet. Most water-soluble vitamins are used to make coenzymes.
28. Which vitamin is not present in plants?
Vitamin B12 is not present in plants.
29. Which vitamin is involved in cholesterol synthesis?
Pantothenic acid (vitamin B5) is involved in cholesterol synthesis. 30. Why does a deficiency of vitamin C lead to weakened connective tissue?
Vitamin C (ascorbic acid) is essential for the synthesis of hydroxyproline and hydroxylysine, which are used in collagen synthesis. Without the hydroxyproline and hydroxylysine, the collagen has reduced hydrogen bonding between the polypeptides in the triple helices, so the helices can unravel. Collagen is the main component of connective tissue.
31. Is beta-carotene the same thing as vitamin A? Explain.
One beta-carotene molecule can be cleaved by oxidation of the central double bond to form two molecules of vitamin A. Thus, beta-carotene is not vitamin A, but is an excellent source of vitamin A.
32. Why is vitamin D not technically a vitamin?
Vitamin D is not technically a vitamin because we can biosynthesize it from 7- dehydrocholesterol in the presence of direct sunlight.
33. Why can a deficiency of vitamin K be dangerous going into surgery?
A deficiency of vitamin K can be dangerous going into surgery because vitamin K is required for synthesis of the zymogens used for blood clotting. This reduced clotting ability could produce excessive bleeding during surgery.