Stanford University HRPP Informed Consent Checklist Medical: Expedited/Minimal Risk CHK-C2 1/3
Protocol ID: Protocol Director:
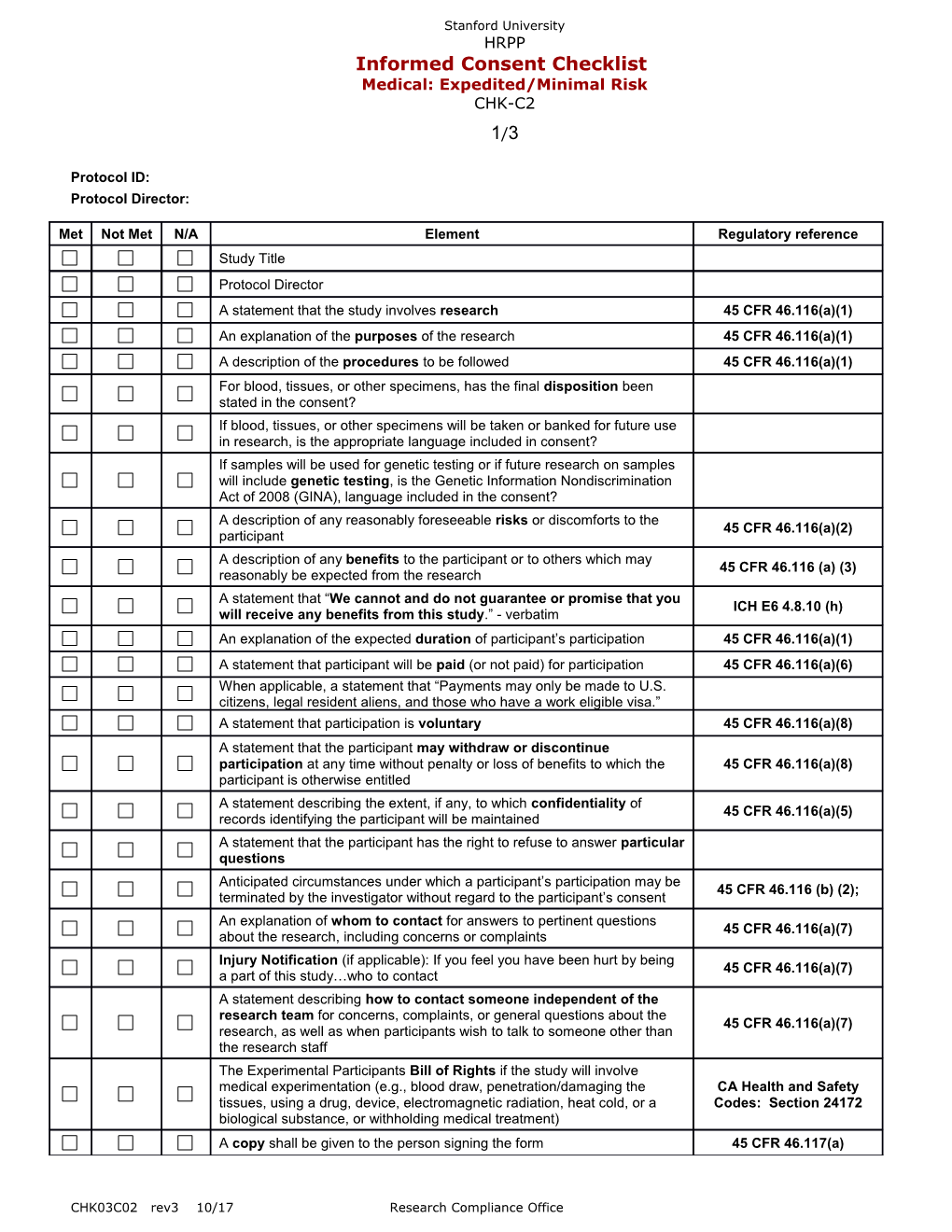
Met Not Met N/A Element Regulatory reference Study Title Protocol Director A statement that the study involves research 45 CFR 46.116(a)(1) An explanation of the purposes of the research 45 CFR 46.116(a)(1) A description of the procedures to be followed 45 CFR 46.116(a)(1) For blood, tissues, or other specimens, has the final disposition been stated in the consent? If blood, tissues, or other specimens will be taken or banked for future use in research, is the appropriate language included in consent? If samples will be used for genetic testing or if future research on samples will include genetic testing, is the Genetic Information Nondiscrimination Act of 2008 (GINA), language included in the consent? A description of any reasonably foreseeable risks or discomforts to the 45 CFR 46.116(a)(2) participant A description of any benefits to the participant or to others which may 45 CFR 46.116 (a) (3) reasonably be expected from the research A statement that “We cannot and do not guarantee or promise that you ICH E6 4.8.10 (h) will receive any benefits from this study.” - verbatim An explanation of the expected duration of participant’s participation 45 CFR 46.116(a)(1) A statement that participant will be paid (or not paid) for participation 45 CFR 46.116(a)(6) When applicable, a statement that “Payments may only be made to U.S. citizens, legal resident aliens, and those who have a work eligible visa.” A statement that participation is voluntary 45 CFR 46.116(a)(8) A statement that the participant may withdraw or discontinue participation at any time without penalty or loss of benefits to which the 45 CFR 46.116(a)(8) participant is otherwise entitled A statement describing the extent, if any, to which confidentiality of 45 CFR 46.116(a)(5) records identifying the participant will be maintained A statement that the participant has the right to refuse to answer particular questions Anticipated circumstances under which a participant’s participation may be 45 CFR 46.116 (b) (2); terminated by the investigator without regard to the participant’s consent An explanation of whom to contact for answers to pertinent questions 45 CFR 46.116(a)(7) about the research, including concerns or complaints Injury Notification (if applicable): If you feel you have been hurt by being 45 CFR 46.116(a)(7) a part of this study…who to contact A statement describing how to contact someone independent of the research team for concerns, complaints, or general questions about the 45 CFR 46.116(a)(7) research, as well as when participants wish to talk to someone other than the research staff The Experimental Participants Bill of Rights if the study will involve medical experimentation (e.g., blood draw, penetration/damaging the CA Health and Safety tissues, using a drug, device, electromagnetic radiation, heat cold, or a Codes: Section 24172 biological substance, or withholding medical treatment) A copy shall be given to the person signing the form 45 CFR 46.117(a)
CHK03C02 rev3 10/17 Research Compliance Office Met Not Met N/A Documentation of Informed Consent
Informed consent shall be documented by the use of a written consent 45 CFR 46.117(a) form approved by the IRB and signed by participant, or participant’s legally authorized representative Two parental permission signature lines / authority to sign for participant 45 CFR 46.408 Signature and dateline for the Person Obtaining Consent Signature and dateline for the Witness if accompanied by a short form consenting process Waiver of Documentation of Informed Consent (Online consent or 45 CFR 46.117(c)(2) Information sheet) – not required outside research Waiver of Documentation of Informed Consent (Online consent or 45 CFR 46.117(c)(1) Information sheet) – only link Alteration of Informed Consent (e.g. Research uses Deception) 45 CFR 46.116(d)
Met Not Met N/A Additional Elements A disclosure of appropriate alternative procedures or courses of 45 CFR 116(a)(4) treatment, if any, that might be advantageous to the participant A statement that the particular treatment or procedure may involve risks to 45 CFR 46.116(b)(1) the participant which are currently unforeseeable A statement that significant new findings developed during the course of the research which may relate to the participant’s willingness to continue 45 CFR 46.116(b)(5) participation will be provided to the participant The consequences of a participant’s decision to withdraw from the 45 CFR 46.116(b)(4) research (if applicable) An explanation as to whether any compensation and/or any medical treatments are available if injury occurs and, if so, what they consist of, or 45 CFR 46.116(a)(6) where further information can be obtained Any additional costs to the participant that may result from participation in 45 CFR 46.116(b)(3) the research The anticipated prorated payment, if any, to the participant for participating ICH E6 4.8.10 (k) in the study
Met Not Met N/A HIPAA Purpose Are appropriate HIPAA elements included? Sign HIPAA Authorization Revocation Contact Info Waiver of Authorization PHI Who May Use/Disclose Alteration of Authorization (Online consent/Information sheet) Who May Receive/Use PAIRE Waiver of Authorization for Recruitment Expiration Date Limited Access to medical record
Met Not Met N/A Additional Items
Include protocol approval and expiration dates Place for participant to indicate/initial if they are participating in other research studies If a consultative/financial relationship exists, does the consent include this disclosure? If the study involves video or audio taping, does the consent include a statement as to what will become of tapes after use, e.g., shown at scientific meetings, erased Place for participant to indicate/initial explicit consent to be taped Place for participant to indicate/initial explicit consent for tapes to be used Place for participant to indicate/initial explicit consent for identity to be made known from the audio/video tapes Template language: MRI (Magnetic Resonance Imaging) including tattoo risk language Is an assent document included? 46.404/50.51 one parent signature statement Stanford University HRPP Informed Consent Checklist Medical: Expedited/Minimal Risk CHK-C2 3/3
Met Not Met N/A Additional Items For studies that include adults and minors, is an optional combined document (adult and parental consent) appropriate? CoC: NIH funded study (or funded by one of the NIH institutes) and collecting identifiable or coded information or generating individual‐level genetic Information, CoC statement in the consent form.
CHK03C02 rev3 10/17 Research Compliance Office