CH081 Introduction to the Laboratory
Name ______
Part I: Using the Balance
1. Weigh three small objects (pennies or paper clips) one at a time, recording the mass of each object as you weigh it. Be sure to include the units! Mass of Object #1 Mass of Object #2 Mass of Object #3 Sum of the 3 masses
Now weigh these three objects together: Mass of 3 objects ______
Comment on your results. Is this what you expected?
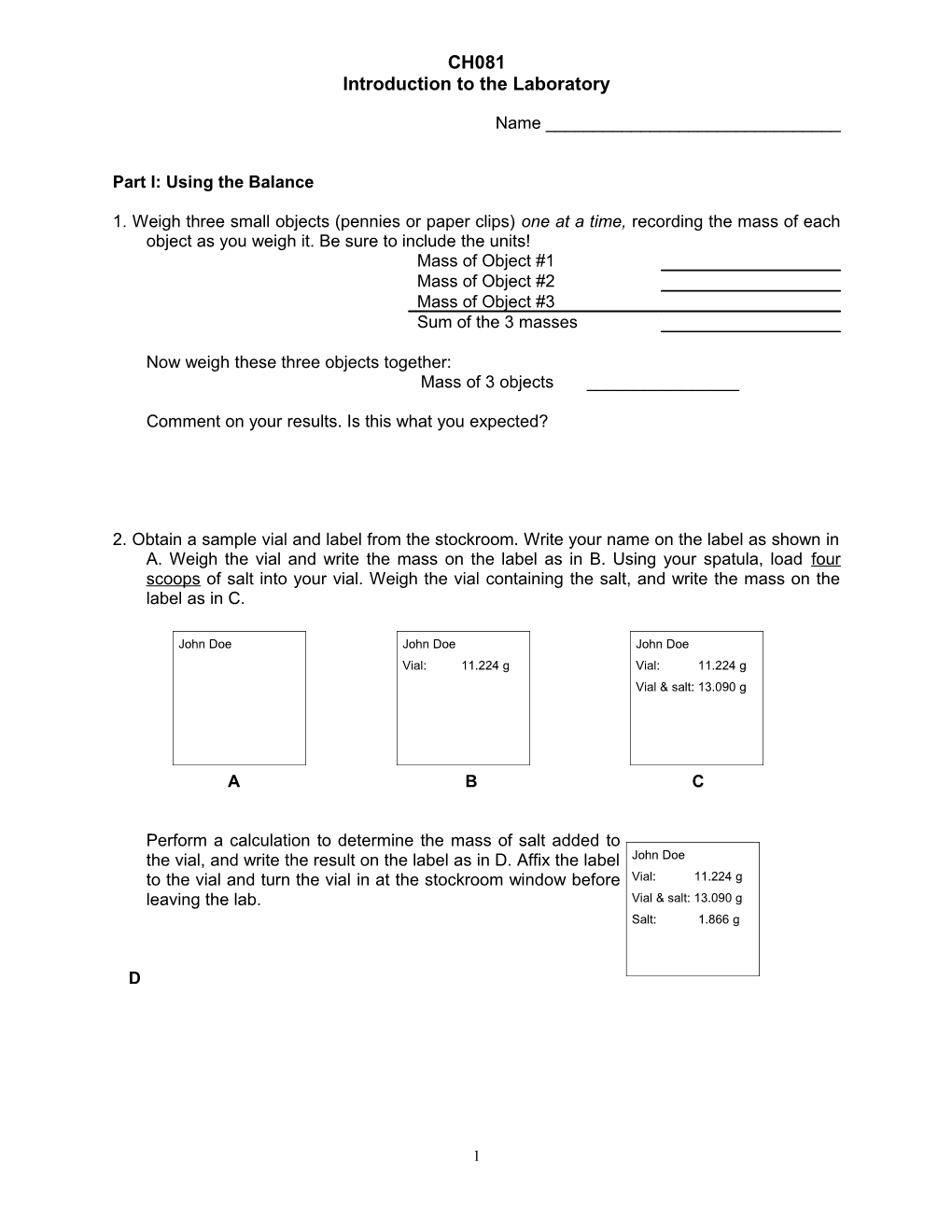
2. Obtain a sample vial and label from the stockroom. Write your name on the label as shown in A. Weigh the vial and write the mass on the label as in B. Using your spatula, load four scoops of salt into your vial. Weigh the vial containing the salt, and write the mass on the label as in C.
John Doe John Doe John Doe Vial: 11.224 g Vial: 11.224 g Vial & salt: 13.090 g
A B C
Perform a calculation to determine the mass of salt added to the vial, and write the result on the label as in D. Affix the label John Doe to the vial and turn the vial in at the stockroom window before Vial: 11.224 g leaving the lab. Vial & salt: 13.090 g Salt: 1.866 g
D
1 CH081 Introduction to the Laboratory
Part II: Limits of Measurements
The equipment that you checked into your lab drawer will help you to observe the behavior of substances. Often, we will prefer to make our observations in the form of measurements, that is, we will use an instrument to tell us something about a substance. In this part of the lab, you will look at:
graduated cylinder metric ruler thermometer
The most important thing to remember about a measurement is that the last digit in a measurement is estimated. That is, there is some uncertainty associated with every measurement that we make. Nevertheless, in terms of significant figures, the last digit is considered to be significant as so is recorded as such.
For instance on the scale below the units are divided into 10 equal spaces. This means that the
smallest increment measures 0.1 unit, which is 1 place past the decimal point. We will be using a "rule of thumb." We will divide this smallest space by 2 to find our limit for any particular scale. 0.1 / 2 = 0.05 unit. So our estimate will be recorded to 2 places past the decimal point, or to 0.01 unit. a. Take your graduated cylinder and record the smallest increment (the smallest space) in decimal form, including units. ______b. Using the rule of thumb, what is the limit of your measurement using this graduated cylinder? ______c. Repeat this process using the ruler and the thermometer you find in your drawer. Record the room temperature and the length of a paper clip using the proper number of significant figures and units for each. ROOM TEMPERATURE ______
LENGTH OF PAPER CLIP ______
Obtain your instructor's or lab assistant's initials here when you are finished.
______
2