Chemistry 11 Review Unit 6
Chemistry 11 Name ______Date ______Review of Unit 6
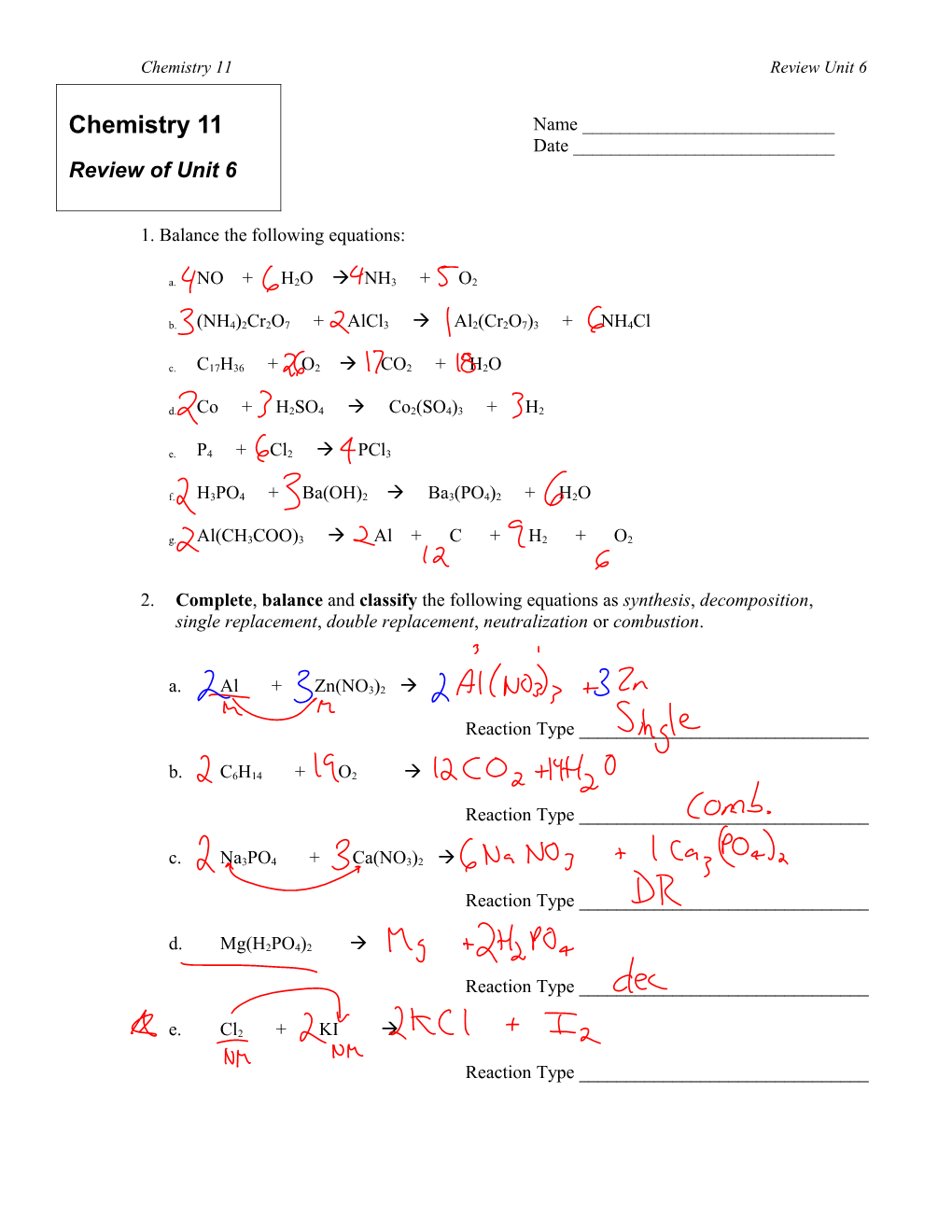
1. Balance the following equations:
a. NO + H2O NH3 + O2
b. (NH4)2Cr2O7 + AlCl3 Al2(Cr2O7)3 + NH4Cl
c. C17H36 + O2 CO2 + H2O
d. Co + H2SO4 Co2(SO4)3 + H2
e. P4 + Cl2 PCl3
f. H3PO4 + Ba(OH)2 Ba3(PO4)2 + H2O
g. Al(CH3COO)3 Al + C + H2 + O2
2. Complete, balance and classify the following equations as synthesis, decomposition, single replacement, double replacement, neutralization or combustion.
a. Al + Zn(NO3)2
Reaction Type ______
b. C6H14 + O2
Reaction Type ______
c. Na3PO4 + Ca(NO3)2
Reaction Type ______
d. Mg(H2PO4)2
Reaction Type ______
e. Cl2 + KI
Reaction Type ______Chemistry 11 Review Unit 6
f. Al + F2
Reaction Type ______
g. Zn + Cu(ClO3)2
Reaction Type ______
h. Co(NO3)3 + (NH4)2C2O4
Reaction Type ______
i. C12H25OH + O2
Reaction Type ______
j. Sr(OH)2 + HNO3
Reaction Type ______
k. V + O2
(Assume combining capacity of V is 5+)
Reaction Type ______
l. Rb3AsO4
Reaction Type ______
m. CsOH + H3PO4
Reaction Type ______
n. Ni(NO3)2 + (NH4)3PO4
Reaction Type ______
o. Al + H2CO3
Reaction Type ______
p. MgSO4 + AgNO3
Reaction Type ______Chemistry 11 Review Unit 6
3. State whether each of the following are exothermic or endothermic.
a. HCl + 432 kJ H + Cl Answer ______
b. C12H22O11 + 12 O2 12CO2 + 11H2O + 5638 kJ Answer ______
c. H2O(l) H2O(g) Answer ______
d. A + B ) J k (
y g r e AB n E Reaction Proceeding
Answer ______
e. C + D CD H= +65.7 kJ Answer ______
f. E + F G + H= - 437 kJ Answer ______
4. In an exothermic reaction, the surroundings get (warmer/cooler) ______.
5. Define enthalpy
6. Why does a light stick glow brighter when it is heated?
7. Which reaction will be faster? 6M HCl with zinc or 3 M HCl with zinc
8. Does concentration increase the rate of reaction A or B. Reaction A 2Na(s) + SrCrO4(s) → Sr(s) + Na2CrO4(s) Reaction B Ba(OH)2 (g) + MgCl2(aq) → Mg(OH)2(aq) + BaCl2(aq) 9. How does surface area affect reaction rate? Give an example of a reaction that IS NOT affected by surface area. Chemistry 11 Review Unit 6
10. Use the following Potential Energy Diagram to answer the questions below: 100
ABC 80 ) J k ( 60 y AB + C g r e n E l a i 40 t n e t o P A + BC 20
0 Progress of Reaction
a) Determine the Activation Energy for the forward reaction... ______kJ
b) Determine the Activation Energy for the reverse reaction.... ______kJ
c) What is the Enthalpy Change (H) for the forward reaction?.. ______kJ
d) What is the Enthalpy Change (H) for the reverse reaction?.. ______kJ
e) The forward reaction is ______thermic.
f) The reverse reaction is ______thermic.
g) Which species or set of species forms the Activated Complex? ______
h) What is the formula for the activated complex? ______
11. State the meaning of Activation Energy Chemistry 11 Review Unit 6
12. Use the following Potential Energy Diagram to answer the questions below:
Potential Energy ABC 300
250
200 A + BC 150
100 AB + C 50
0 0.0 0.2 0.4 0.6 0.8 1.0 1.2 Progress of Reaction
a) Determine the Activation Energy for the forward reaction... ______kJ
b) Determine the Activation Energy for the reverse reaction.... ______kJ
c) What is the Enthalpy Change (H) for the forward reaction?..______kJ
d) What is the Enthalpy Change (H) for the reverse reaction?..______kJ
e) The forward reaction is ______thermic.
f) The reverse reaction is ______thermic.
g) Which species or set of species forms the Activated Complex? ______