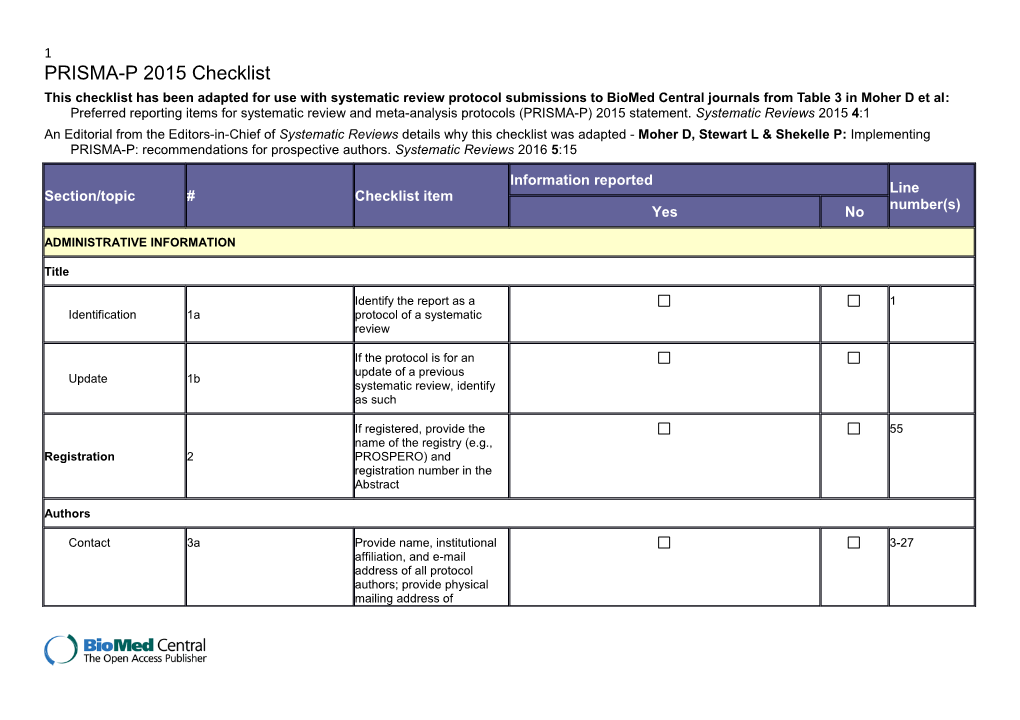
1 PRISMA-P 2015 Checklist This checklist has been adapted for use with systematic review protocol submissions to BioMed Central journals from Table 3 in Moher D et al: Preferred reporting items for systematic review and meta-analysis protocols (PRISMA-P) 2015 statement. Systematic Reviews 2015 4:1 An Editorial from the Editors-in-Chief of Systematic Reviews details why this checklist was adapted - Moher D, Stewart L & Shekelle P: Implementing PRISMA-P: recommendations for prospective authors. Systematic Reviews 2016 5:15
Information reported Line Section/topic # Checklist item number(s) Yes No
ADMINISTRATIVE INFORMATION
Title
Identify the report as a 1 Identification 1a protocol of a systematic review
If the protocol is for an update of a previous Update 1b systematic review, identify as such
If registered, provide the 55 name of the registry (e.g., Registration 2 PROSPERO) and registration number in the Abstract
Authors
Contact 3a Provide name, institutional 3-27 affiliation, and e-mail address of all protocol authors; provide physical mailing address of 2 Information reported Line Section/topic # Checklist item number(s) Yes No
corresponding author
Describe contributions of 277-81 Contributions 3b protocol authors and identify the guarantor of the review
If the protocol represents an amendment of a previously completed or published protocol, identify as such Amendments 4 and list changes; otherwise, state plan for documenting important protocol amendments
Support
Indicate sources of financial 275-6 Sources 5a or other support for the review
Provide name for the review 275-6 Sponsor 5b funder and/or sponsor
Describe roles of funder(s), 275-90 Role of sponsor(s), and/or 5c sponsor/funder institution(s), if any, in developing the protocol
INTRODUCTION
Rationale 6 Describe the rationale for 67-76 the review in the context of 3 Information reported Line Section/topic # Checklist item number(s) Yes No
what is already known
Provide an explicit 74-6 statement of the question(s) the review will address with Objectives 7 reference to participants, interventions, comparators, and outcomes (PICO)
METHODS
Specify the study 85-146 characteristics (e.g., PICO, study design, setting, time frame) and report Eligibility criteria 8 characteristics (e.g., years considered, language, publication status) to be used as criteria for eligibility for the review
Describe all intended 147-60 information sources (e.g., electronic databases, Information sources 9 contact with study authors, trial registers, or other grey literature sources) with planned dates of coverage
Search strategy 10 Present draft of search 161-78 strategy to be used for at Additional file 2 least one electronic 4 Information reported Line Section/topic # Checklist item number(s) Yes No
database, including planned limits, such that it could be repeated
STUDY RECORDS
Describe the mechanism(s) 179-81 that will be used to manage Data management 11a records and data throughout the review
State the process that will 182-93 be used for selecting studies (e.g., two independent Selection process 11b reviewers) through each phase of the review (i.e., screening, eligibility, and inclusion in meta-analysis)
Describe planned method of 194-203 extracting data from reports (e.g., piloting forms, done Data collection 11c independently, in duplicate), process any processes for obtaining and confirming data from investigators
List and define all variables 196-9 for which data will be sought Additional file 2 (e.g., PICO items, funding Data items 12 sources), any pre-planned data assumptions and simplifications 5 Information reported Line Section/topic # Checklist item number(s) Yes No
List and define all outcomes 196-9 for which data will be Additional file 2 Outcomes and sought, including 13 prioritization prioritization of main and additional outcomes, with rationale
Describe anticipated 204-18 methods for assessing risk of bias of individual studies, Risk of bias in including whether this will 14 individual studies be done at the outcome or study level, or both; state how this information will be used in data synthesis
DATA
Synthesis Describe criteria under 15a which study data will be quantitatively synthesized
15b If data are appropriate for quantitative synthesis, describe planned summary measures, methods of handling data, and methods of combining data from studies, including any planned exploration of consistency (e.g., I 2, Kendall’s tau) 6 Information reported Line Section/topic # Checklist item number(s) Yes No
Describe any proposed additional analyses (e.g., 15c sensitivity or subgroup analyses, meta-regression)
If quantitative synthesis is 219-35 not appropriate, describe 15d the type of summary planned
Specify any planned assessment of meta- bias(es) (e.g., publication Meta-bias(es) 16 bias across studies, selective reporting within studies)
Describe how the strength 237-43 Confidence in 17 of the body of evidence will cumulative evidence be assessed (e.g., GRADE)