Kelce Gerlits Osmosis and Diffusion Lab
Processed Data: (Raw Data can be found attached as Appendix A)
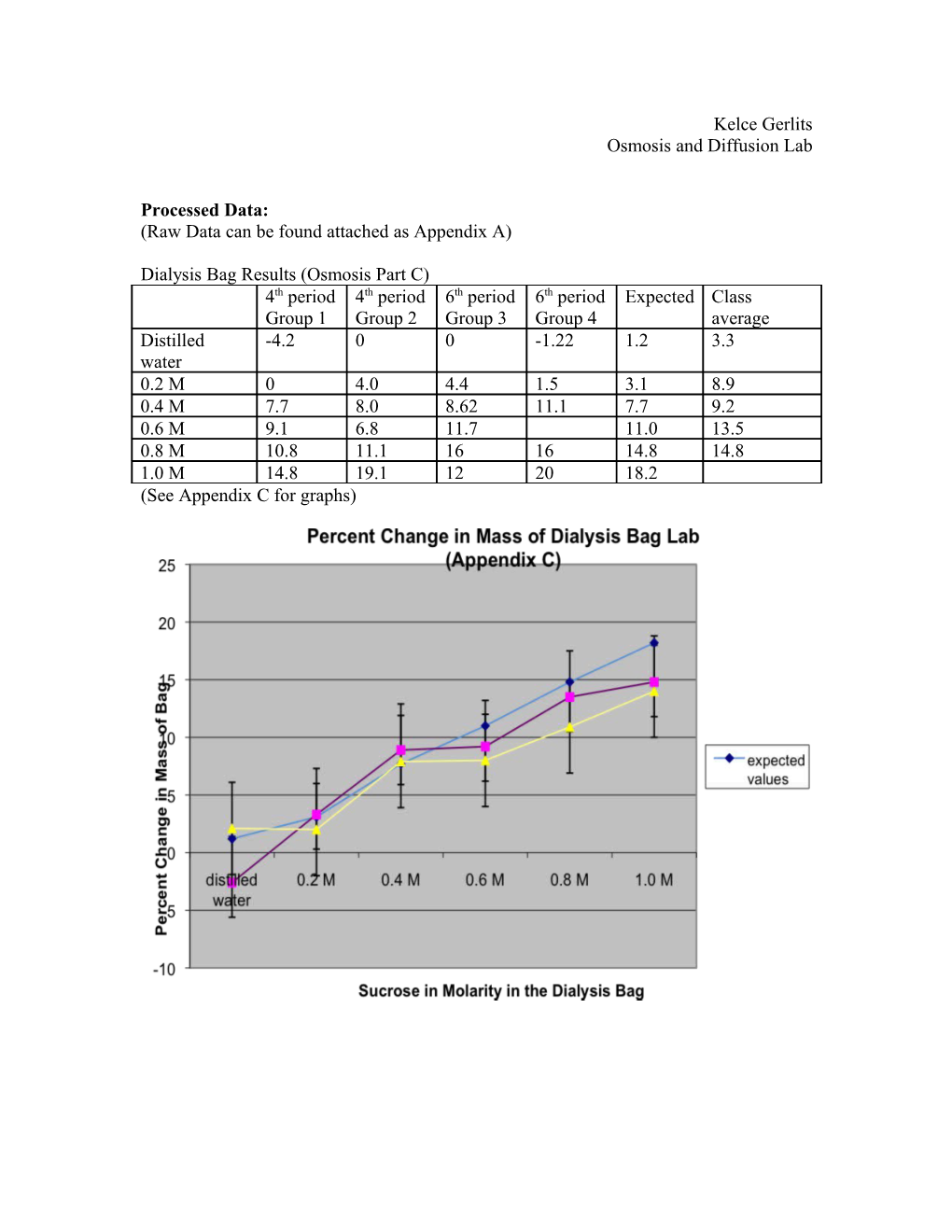
Dialysis Bag Results (Osmosis Part C) 4th period 4th period 6th period 6th period Expected Class Group 1 Group 2 Group 3 Group 4 average Distilled -4.2 0 0 -1.22 1.2 3.3 water 0.2 M 0 4.0 4.4 1.5 3.1 8.9 0.4 M 7.7 8.0 8.62 11.1 7.7 9.2 0.6 M 9.1 6.8 11.7 11.0 13.5 0.8 M 10.8 11.1 16 16 14.8 14.8 1.0 M 14.8 19.1 12 20 18.2 (See Appendix C for graphs) Percent Change in Potato Core Results (Osmosis Part A) Group 1 Group 2 Group 3 Group 4 Expected Class (in %) (in %) (in %) (in %) (in %) average (%) Distilled 13.8 18.8 20 20 21.4 15 water 0.2 M 5.7 7.6 11.1 6.9 8.5 0.4 M 6.4 13.0* -3.7 -4.5 3.9 0.6 M -21.8 -21.6 -30.9 -12.8 -22 0.8 M -25.9 -21.9 -25.5 -25.0 -24 1.0 M -17.9 -27.4 -30.2 -30.2 -30.7 -27.8 *Outlier, due to possible contamination (See Appendix B for graphs)
Cell Osmosis (Osmosis Part B) Before NaCl (Initial) Addition of NaCl Removal of NaCl (Flood) Visible, block-like cell Cell walls are blurry and Cells are not much more walls. pushed together. clear, however the block and wideness of the cell has returned. (Magnification 40x) (See Appendix A for sketches)
Data Analysis: (Osmosis Part C) The experiment titled Dialysis Bag, was conducted with a small plastic bag filled with sucrose and tied with strings at the end of each side, then placed into a beaker filled with water. An error that may have occurred during this experiment could be that the bag had a hole in it, or the strings weren’t tied tightly enough, therefore allowing the sucrose in the bag to seep into the water and ruining the experiment. (See Appendix C for graph of the data)
(Osmosis Part A) The experiment using a potato core was conducted by first coring a potato, then placing it in several beakers with different concentrations of sucrose, ranging from distilled water (no sucrose) to 1M of sucrose solution. Error found in this experiment was when the solutions were being poured into beakers. Due to the amount of solution, beakers, and people in the process of conducting this experiment, solutions could have been mixed due to the mixing of measuring items, which could have tampered and ruined the outcomes of the experiment.
(Osmosis Part B) Error found in this experiment with the onion cells was the process of preparing the slide. Some of the onion skins could not have been sliced enough which did not allow much observation, or the slide could have been messed up as well with the addition of water and/or NaCl.
Conclusion and Evaluation:
(Osmosis Part C) The data in this lab indicates that due to osmosis, water will diffuse into the low concentration gradient found in the plastic baggy filled with various levels of sucrose solutions, to the high water concentration gradient of water the baggy was immersed in. For example, as shown in the data table, at .2M sucrose solution in the plastic bag (the selectively permeable membrane), the class found an average of a 3.3% mass increase. Also, when the bag contained a concentration of .8M of sucrose solution, the average mass gain was 13.5%. A limitation found in this experiment would be time, because our group rushed to finish this section of the lab before class was over, and ended up ending two or so minutes early. This would not skew the data greatly, however it still may have. Some weaknesses found were shared materials, and the materials used. The solutions, beakers, and funnels used in this experiment all ran the risk of being cross contaminated, which could be changed by giving each group their own set of instruments. Another weakness found was the risk of the plastic baggy actually leaking due to the string not being tied tight enough or a whole in the baggy. This could be fixed by finding different materials that still provide the same purpose.
(Osmosis Part A) The expected values, along with the majority of the class values, show that potato cores absorb liquid under the concentration of about .2M of sucrose, because after .2M on the table, the percentages are negative. For example, at .2M of sucrose, the potato’s mass increase according to the class average was 8.5%, whereas the potato’s mass increase at . 6M of sucrose solution was -22%. These numbers help us further understand osmosis in a real life situation. The potato is a root, which means it grows underground, and so when it is watered it has to absorb the water faster than the dirt around it. Proven by the data in this lab, potatoes have a very low molarity of water, so when water is added to the potato and around the potato, it will absorb it up because of the potato’s low water concentration and water’s attraction to low concentrations due to osmosis. A limiting factor found in this lab was the amount of time. This cannot really be helped since we can only devote so much time to labs at school, however if we put aside more days or time to work on this lab, it would eliminate the problem. A weakness found in this lab is the lack of the group’s own solutions and beakers, which runs the risk of cross contamination. One way to improve this would be to give every group each their own separate kit.
(Osmosis Part B)
This lab displays the osmosis process in an onion cell. The onion cells were observed as normal, then immersed in a solution of NaCl, and then observed again. The difference in the cells before and after was great, the cells shriveled upon entry of NaCl, due to diffusion. However, when the cells were flushed with water again after the NaCl was added, the cells returned to normal size due to osmosis. This lab can be used with the same observation as the potato lab. The onion is also a root as well, so it would have to absorb the water before the dirt around it did. This lab also shows that the onion easily takes water into its cells before any other substances such as NaCl. A weakness found in this lab was that the class viewed the cell at only 40x, versus the recommended 100x. However, the process could still be easily viewed and observed under 40x.