Supporting Information for
Thermo- and pH -responsive fluorescence behavior of sulfur functionalized detonation nanodiamond-poly (N-isopropylacrylamide)
Siheng Su[1], Junhua Wei[1] , Kun Zhang[2], and Jingjing Qiu[1]*
[1]Department of Mechanical Engineering, Texas Tech University, 2500 Broadway, P.O. Box 43061, Lubbock, TX 79409, United States. [2] Department of Industrial Engineering, Texas Tech University, 2500 Broadway, P.O. Box 43061, Lubbock, TX 79409, United States.
*Corresponding Author: E-mail: [email protected]
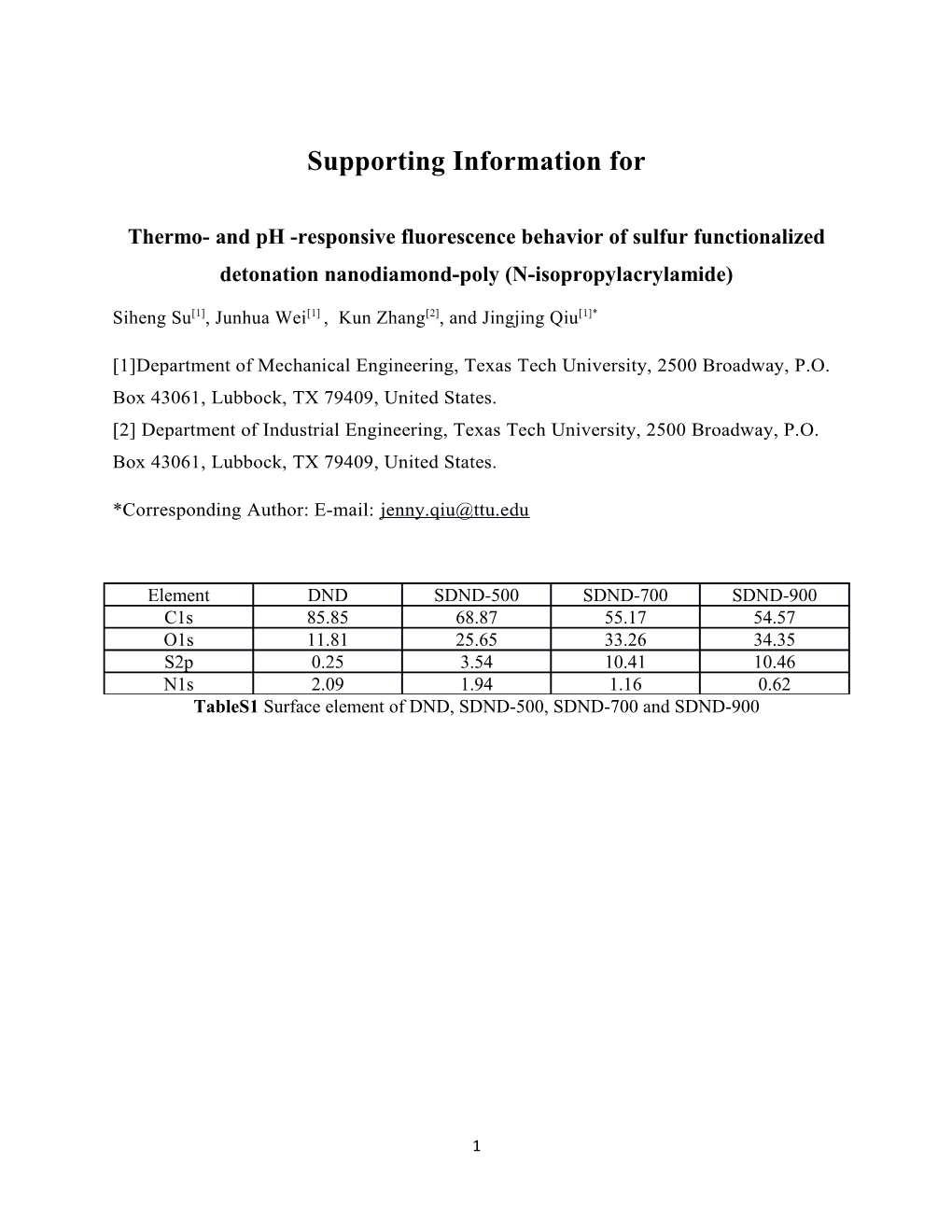
Element DND SDND-500 SDND-700 SDND-900 C1s 85.85 68.87 55.17 54.57 O1s 11.81 25.65 33.26 34.35 S2p 0.25 3.54 10.41 10.46 N1s 2.09 1.94 1.16 0.62 TableS1 Surface element of DND, SDND-500, SDND-700 and SDND-900
1 Figure S1: TGA of BDS, DND and DND/BDS mixture. According to the TGA results, the condensation of BDS on the surface of DND can be excluded since BDS stars decomposition at its melting point (~78 ̊C) and it is totally decomposed around 300 ̊C. Therefore, the resultants are sulfur modified DND after 2hr air oxidation.
2 Scheme S1: Mechanism of sulfur functionalized nanodiamond
3 Quantum yield of SDNDs calculation
Single point method[1] was used to determine the quantum yields of SDNDs, and quinine sulfate in 0.1M sulfuric acid with quantum yield 54% was used as standard. All the tested samples were dissolved in DI water and their photoluminescence (PL) emission spectra were obtained by a FluoroMax-3 from 360nm to 600nm at excitation 340nm, while the absorbance was measured by a Lambda 1050 from PerkinElmer. The absolute value of quantum yield is calculated by the following equation:
The subscripts ST and X represent standard sample and test sample, is the fluorescence quantum yield, is the gradient from the plot of integrated fluorescence intensity vs. absorbance, and is the refractive index of the solvent. Since water is used as solvent the all the samples, .
Figure S2. Absorption (a) and emission (excitation: 340nm) (b) of SDND-500, SDND- 700, and SDND-900
4 PL Integrate Absorbance Quantum Yield (%) Quinine Sulfate 3017400224 0.310931 54 SDND500 53988508 0.086849 3.45 SDND 700 62119965 0.099138 3.49 SDND 900 96978738 0.096571 5.59 Table S2: PL integrate, absorbance and quantum yield of all the samples
Figure S3. Absorbance spectrum (a) of DND, and emission spectra (b) of DND-900. It indicated that DND-900 has no fluorescence under different wavelength excitation which demonstrates the fluorescence of SDNDs comes from sulfur functional groups during BDS pyrolysis.
5 FigureS4. Dynamic radius (a) and zeta potential (b) of DND and SDND. It shows that the dispersion and stability of SDND-900 in aqueous solution are improved compared to DND.
Reference
[1] Wang, Z.;Xu, C.; Liu, C. Surface modification and intrinsic green fluorescence emission of a detonation nanodiamond. Journal of Materials Chemistry C 2013, 1, 6630-6636.
6