Additional File 3. Risk of bias and quality assessments.
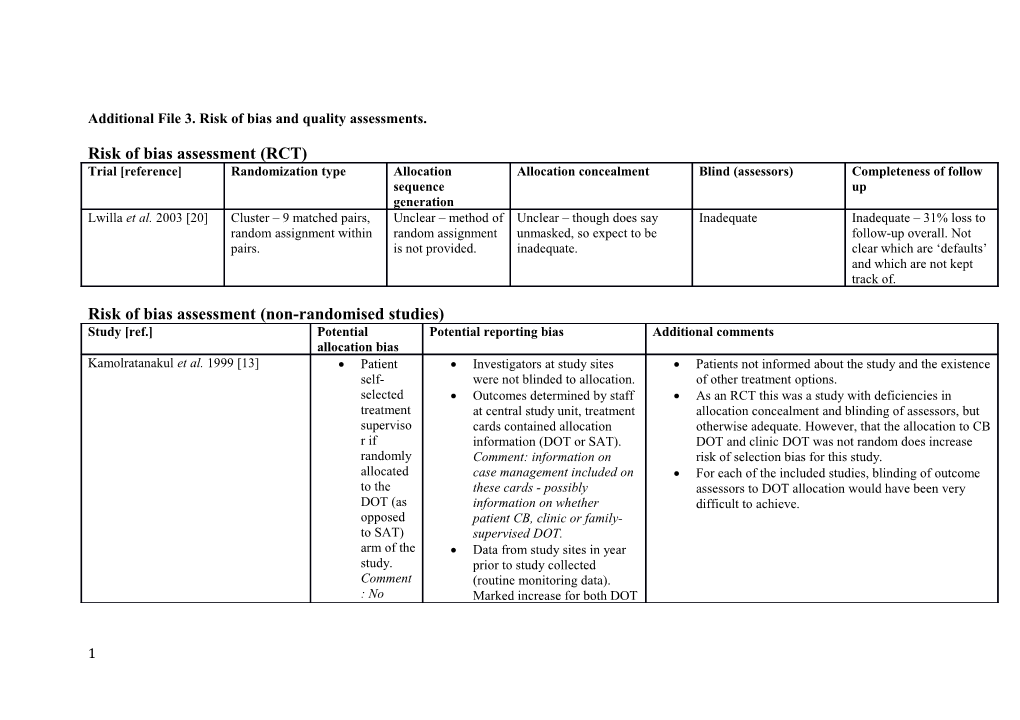
Risk of bias assessment (RCT) Trial [reference] Randomization type Allocation Allocation concealment Blind (assessors) Completeness of follow sequence up generation Lwilla et al. 2003 [20] Cluster – 9 matched pairs, Unclear – method of Unclear – though does say Inadequate Inadequate – 31% loss to random assignment within random assignment unmasked, so expect to be follow-up overall. Not pairs. is not provided. inadequate. clear which are ‘defaults’ and which are not kept track of.
Risk of bias assessment (non-randomised studies) Study [ref.] Potential Potential reporting bias Additional comments allocation bias Kamolratanakul et al. 1999 [13] Patient Investigators at study sites Patients not informed about the study and the existence self- were not blinded to allocation. of other treatment options. selected Outcomes determined by staff As an RCT this was a study with deficiencies in treatment at central study unit, treatment allocation concealment and blinding of assessors, but superviso cards contained allocation otherwise adequate. However, that the allocation to CB r if information (DOT or SAT). DOT and clinic DOT was not random does increase randomly Comment: information on risk of selection bias for this study. allocated case management included on For each of the included studies, blinding of outcome to the these cards - possibly assessors to DOT allocation would have been very DOT (as information on whether difficult to achieve. opposed patient CB, clinic or family- to SAT) supervised DOT. arm of the Data from study sites in year study. prior to study collected Comment (routine monitoring data). : No Marked increase for both DOT
1 Study [ref.] Potential Potential reporting bias Additional comments allocation bias informati and SAT arms during the on as to study relative to treatment whether outcome data from the there was preceding year. any input from health professio nals or others on this choice. Kironde & Meintjies 2002 [26] Patients Interviewers included DOT Study areas recruited randomly from 45 PHC facilities are supervisors. Comment; interviewers (secondary and tertiary units excluded, random selection from normally not blinded to allocation. an SPSS algorithm - this seems adequate). given the Patients with missing data were For new patients, rural residence was associated with a higher option of excluded. Comment; could this mean chance of treatment success. clinic or loss to follow-up is under-estimated, Multiple sociodemographic factors considered in analysis – CB DOT even though these data are not broken allocation of treatment supervisor was one of these, others and told down for supervision type default is included age, sex, education level, whether living on a to make a reported as 18.7% overall. disability pension etc. choice Prospective study, thus more controlled than a retrospective between study. these two with the advice of the clinic nurse. Comment ; What
2 Study [ref.] Potential Potential reporting bias Additional comments allocation bias advice does the clinic nurse give? No criteria stated to guide allocation . CB DOT supporter lives within a ‘reasonable proximity’ of the patient, patient goes to the provider. Comment; what is considered reasonable, does greater distance affect outcomes?
3 Study [ref.] Potential Potential reporting bias Additional comments allocation bias Miti et al. 2003 [27] Twapia (control) Programmatic change. Comment; risk Prospective trial, thus more controlled than a retrospective and Chipulukusu of reporting bias, existing program, study. For example, a new sputum transport system was (intervention) research as a result of invitation to introduced but was provided to both intervention and control compounds; “both join a ‘Community TB care in Africa’ arm communities are project. Limited sociodemographic commentary but age for both economically Training applied to volunteers from groups very similar (intervention: mean age of 32.4 years in deprived, with intervention area (3 days) and to intervention area and 32.5 years in control area). poor living nurses from both areas. Comment; conditions, and duration of nurse training and content both communities not elaborated upon. are served by a No information on whether people small health analysing data were blinded to centre and have allocation. primary schools” Comment; no quantitative data provided in the paper to substantiate comparability. In Twapia (control) ratio of male:female was close to 1:1 while it was 1:2 in Chipulukusu (internvention). Comment: no specific mention in the paper
4 Study [ref.] Potential Potential reporting bias Additional comments allocation bias whether this difference impacted on results. Initially higher patient numbers in Twapia (control) relative to Chipulukusu (intervention). Comments: authors attribute to community adjustment to programmatic change as TB services in intervention area newly directed through HIV Home Care Programme. Unclear if this affected overall result. Rate of HIV co- infection in CB DOT and clinic DOT populations not specified or
5 Study [ref.] Potential Potential reporting bias Additional comments allocation bias whether there was an overall difference between the two. Death rates are not significantly different (22% for CB DOT versus 19% for clinic DOT). Last section of discussion mentions that: “Few tuberculosis patients in the Chipulukusu township who were not previously registered with the HCP [home care programme] opted for the daily visit by community home care volunteers. Instead, many opted for daily visits to the health centre to receive
6 Study [ref.] Potential Potential reporting bias Additional comments allocation bias their daily medications”. Comment: Not specified what the patient number was opting for clinic DOT and this is not mentioned as an option in the study methodology. Results for CB DOT area essentially presented as an intention-to-treat with CB DOT by the TB programme and investigators. Niazi and Al-Delaimi 2003 [28] Allocation to one Socio-demographic reporting by NGO Specific DOT training given to NGO volunteers, though no group or the other volunteers, while outcome and mention if training provided to nurses or not. by TB coordinator compliance by TB coordinator. Did not measure other factors that may impact on compliance according to order Comment: prefer to have same person such as time to reach the health facility, waiting time, of attendance report or cross-checking of data satisfaction with patient care received. Comment: extraction. Authors report that no sociodemographic factors influenced potential risk of No information on blinding to results. modifying treatment allocation during data entry Appropriate randomisation (as opposed to sequential allocation. and analysis. allocation) would be helpful in strengthening results. Note that
7 Study [ref.] Potential Potential reporting bias Additional comments allocation bias Some inaccuracies in the paper, for authors state in their discussion, “..because patients were example stating that 83% cure rate randomly assigned to the 2 groups…”, this is not the case. exceeds the 85% standard.
Nirupa et al. 2004 [31] Supervisor Not intention-to-treat (phone call at 2 Patients assigned to different treatment groups had similar selected by months to establish who was sociodemographic factors (based on univariate analysis doctors. supervising therapy). reported). Comment; no Information gaps (couldn't contact Treatment results only provided for new sputum smear- criteria stated to 12% of patients by phone). positive patients (assists with comparability). guide allocation Data computerized and cross-checked. Retrospective data and thus less robust than a prospective Comment; no information on blinding study. to treatment allocation during data entry and analysis. Singh et al. 2004 [29] “Before Senior treatment supervisor visited 2-day training course for CVs, though not mentioned if starting community volunteers (CVs) weekly, additional training provided to government health workers (or treatment, every 2-3 days if issues and doctor if this was required). patients visited once a month. No information Retrospective data and thus less robust than a prospective residing given on supervision of government study. or health workers. working No information on blinding of those in areas evaluating data to allocation type. close to a CV were given the option of receiving DOT from that CV”. Comment
8 Study [ref.] Potential Potential reporting bias Additional comments allocation bias : unclear if location of CVs introduce d a selection bias for the CB DOT arm. Tripathy et al. 2013 [30] “The Investigators not blinded to supervisor All DOT providers (CB DOT and clinic DOT) receive training. patient type. Retrospective data and thus less robust than a prospective chose the Double entry of data mentioned in the study. type of method Comment; not sure if by DOT different people. provider No mention of blinding to treatment in allocation during data entry and consultati analysis. on with the medical officer”. Comment : no criteria stated to guide allocation .
9 GRADE criteria assessment for included studies as relevant to comparison between CB DOT and clinic DOT.
Study [ref.] Starting Study limitations Inconsistency of results (if Indirectness of Imprecision 3 Reporting bias Overall quality quality based (risk of bias) 2 consistent this does not evidence of evidence on study type 1 increase quality) Kamolratanakul Low Possible effect on All studies suggest either This trial compares Imprecise For all studies, Very low for et al. 1999 [13] primary or similar outcomes between DOT to SAT. (95% CI non-blinding of primary and secondary CB DOT and clinic DOT, or However, specific 0.13-2.39 for assessors to secondary outcome. better outcomes from CB results and odd ratio treatment outcome DOT relative to clinic DOT. discussion of (OR)) for allocation (or (downgraded Thus, there is a consistent different DOT primary insufficient due to study result of at least similar providers (family outcome. information on limitations and treatment success from CB member, health Imprecise blinding) imprecision). DOT relative to clinic DOT. worker, community (95% CI introduces a However, there is member) is 0.46-36.55 possibility of substantial inter-study provided, thus for OR) for reporting bias heterogeneity for the considered as direct secondary for both the primary meta-analysis evidence and not a outcome. primary and the (84%). Even so, no studies quality lowering secondary were downgraded in quality issue. outcome. Kironde & Low Possible effect on based on inconsistency of Not a quality Imprecise Blinding of Very low for Meintjies 2002 primary outcome, results. lowering issue. (95% CI outcome primary [26] secondary 0.81-1.75 for assessors to outcome outcome not For secondary outcome, OR) for DOT type is (downgraded assessed for this results for included studies primary very difficult or due to study study. were inconsistent: some outcome. impossible to limitations and showing greater loss to achieve for imprecision). follow-up from clinic DOT, these studies. Secondary some equivalence and one This risk has outcome not study greater loss to follow- been combined assessed
10 Study [ref.] Starting Study limitations Inconsistency of results (if Indirectness of Imprecision 3 Reporting bias Overall quality quality based (risk of bias) 2 consistent this does not evidence of evidence on study type 1 increase quality) Lwilla et al. 2003 High Possible effect on up from CB DOT (see Not a quality Imprecise with the study Low for primary [20] primary and Figure 4 on main paper and lowering issue. (95% CI limitations and secondary secondary additional file 4 for further 0.82-1.65 for assessment outcome outcome. discussion of this result). OR) for when (downgraded primary considering due to study outcome. whether to limitations and Precise (95% downgrade for primary CI 1.39-2.96 study quality outcome for for OR) for due to bias. As imprecision). secondary there are less outcome, than ten studies though not in this meta- entirely clear analysis, funnel which losses plot asymmetry are due to tests are not programme appropriate. and which due to study difficulties. Miti et al. 2003 Low Possible effect on Not a quality Imprecise Very low for [27] primary and lowering issue. (95% CI primary and secondary 0.88-3.05 for secondary outcome. OR) for outcome primary (downgraded outcome. due to study Precise (95% limitations and CI 0.12-0.80 for primary for OR) for outcome for secondary imprecision).
11 Study [ref.] Starting Study limitations Inconsistency of results (if Indirectness of Imprecision 3 Reporting bias Overall quality quality based (risk of bias) 2 consistent this does not evidence of evidence on study type 1 increase quality) outcome.
Niazi and Al- Low Possible effect on Not a quality Precise (same Very low for Delaimi. 2003 primary and lowering issue. result at primary and [28] secondary either end of secondary outcome. CI, 95% CI outcome 1.13-4.89 for (downgraded OR) for due to study primary limitations and outcome. for secondary Imprecise outcome for (95% CI imprecision). 0.43-2.92 for OR) for secondary outcome. Nirupa et al. Low Possible effect on Not a quality Imprecise Very low for 2004 [31] primary and lowering issue. (95% CI primary and secondary 0.92-1.92 for secondary outcome. OR) for outcome primary (downgraded outcome. due to study Precise (95% limitations and CI 0.51-0.92) for primary for secondary outcome for outcome. imprecision). Singh et al. 2004 Low Possible effect on Not a quality Imprecise Very low for [29] primary and lowering issue. (95% CI primary and secondary 0.67-1.66 for secondary
12 Study [ref.] Starting Study limitations Inconsistency of results (if Indirectness of Imprecision 3 Reporting bias Overall quality quality based (risk of bias) 2 consistent this does not evidence of evidence on study type 1 increase quality) outcome. OR) for outcome primary (downgraded outcome. due to study Imprecise limitations and (95% CI imprecision). 0.61-1.72 for OR) for secondary outcome. Tripathy et al. Low Possible effect on Not a quality Precise (95% Low for primary 2013 [30] primary and lowering issue. CI 3.13-6.58 and secondary secondary for OR) for outcome (study outcome. primary limitations outcome. downgrade Precise (95% evidence but CI 0.19-0.63 large effect size for OR) for means that secondary evidence is of outcome. overall low quality). 1 RCTs start as high quality, observational studies start as low quality and this has been applied to the non-randomised studies, even if interventional rather than observational. 2 In spite of existing study limitations, the authors of this systematic review have chosen to include all included studies in the meta-analysis as the selection criteria for the systematic review meant that these included studies had the least risk of bias of all of the studies assessed. 3 Precision judgment based on whether top and bottom of confidence interval were on the same or different sides of the ‘1’ value.
13