CHEM 301 - Dr. Hartel Winthrop University Self Evaluation 2
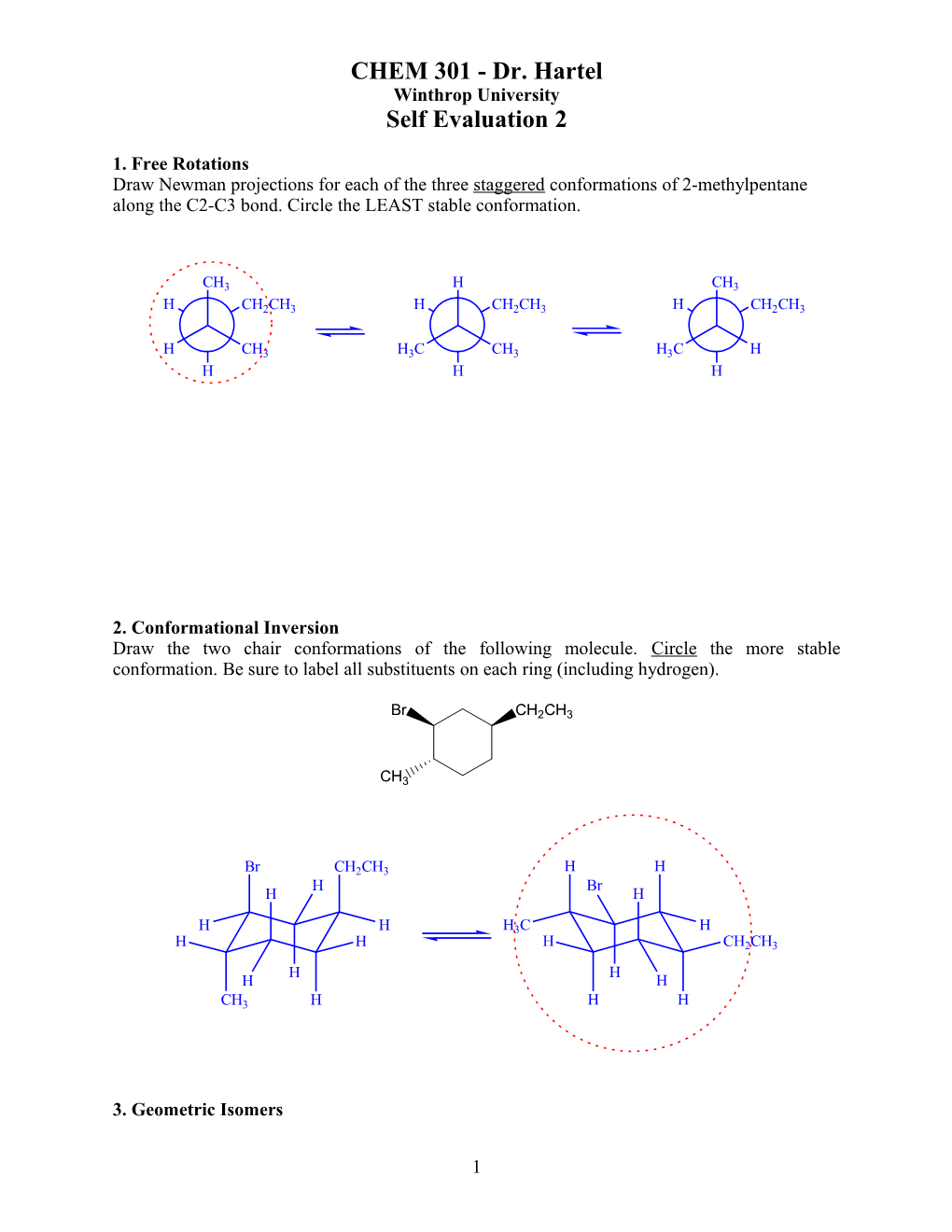
1. Free Rotations Draw Newman projections for each of the three staggered conformations of 2-methylpentane along the C2-C3 bond. Circle the LEAST stable conformation.
CH3 H CH3 H CH2CH3 H CH2CH3 H CH2CH3
H CH3 H3C CH3 H3C H H H H
2. Conformational Inversion Draw the two chair conformations of the following molecule. Circle the more stable conformation. Be sure to label all substituents on each ring (including hydrogen).
Br CH2CH3
CH3
Br CH2CH3 H H H Br H H
H H H3C H H H H CH2CH3 H H H H CH3 H H H
3. Geometric Isomers
1 CHEM 301 - Dr. Hartel Winthrop University Self Evaluation 2
Give the systematic IUPAC names for the following compounds, including proper indication of stereochemistry (cis, trans, E or Z). CH3CHClCH2CH2 CH2CH2CH3 C
C Cl H (E)-3-methyl-4-octene or trans-3-methyl-4-octene (Z)-1,5-dichloro-2-propyl-1-hexene
(CH3)3CCH2CH2CH2
OCH3 OH (E)-7-methoxy-2,4-dimethyl-1,3-heptadiene cis-5-(4,4-dimethylpentyl)cyclooctanol
4. Chirality and Chirality Centers a. Circle all of the chirality centers on the following molecule, which is a potent anti-tumor compound found in a species of marine sponge.
CH3 OCH3 O OCH3 OH N H OH O CH3 CH3 CH3 CH3 HO
O OH
Iriciniastatin OH O b. Circle all of the compounds below that are chiral.
OH
CH3 Br Cl Br Cl Br H H CH3 C C C C C
CH H3C CH3 H3C 3 O CH3 OH
5. Stereochemical Configurations and Relationships
2 CHEM 301 - Dr. Hartel Winthrop University Self Evaluation 2 a. In the boxes provided, assign the stereochemical configuration for each chirality center. In the blank space provided, describe the stereochemical relation between the two molecules.
S S H Br R R Br CH3 H3C H
H3C OCH3 H3C OCH3
Cl CH3 H CH3
H Cl R Stereochemical Relation S Diastereomers
S R
H OH CH3 CH(CH3)2 HO H (CH3)2CH CH3 R S
Stereochemical Relation Enantiomers
b. In the blank space provided, describe the stereochemical relation between the two molecules of each pair.
Cl Cl CHO CH2OH H N H3C H 2 CH3 H OH H OH + + H2N + HO H H OH H H H CH2OH CHO CH3 CH3 H3C CH3 Identical Diastereomers Diastereomers
6. Application of Concepts
3 CHEM 301 - Dr. Hartel Winthrop University Self Evaluation 2 a. Which is more stable, cis-3-hexene or trans-3-hexene? Explain your reasoning.
Free rotation about the C-C σ bonds of trans-3-hexene does not give rise to any significant increase in steric strain. Free rotation about the C-C σ bonds of cis-3-hexene creates a significant increase in steric strain as the two ethyl groups near each other in space. The increased strain makes cis-3-hexene less stable than trans-3-hexene.
b. The antifreeze in your car is primarily ethylene glycol (1,2-ethanediol). Unlike ethane, ethylene glycol is more stable in an eclipsed conformation. Draw the most stable conformation of ethylene glycol, and explain what gives it this unexpected stability.
While there are three eclipsed conformations of ethylene glycol, the one drawn below is the most stable. The unusual stability is the result of an intramolecular hydrogen bond between the H of one alcohol and the O of the other. Hydrogen bonding is a type of bonding interaction, thus an attractive force which stabilizes the molecule. This intramolecular hydrogen bonding would not be available in the other conformation, as the OH groups would be oriented too far away from each other.
H H O O
H H H H
4