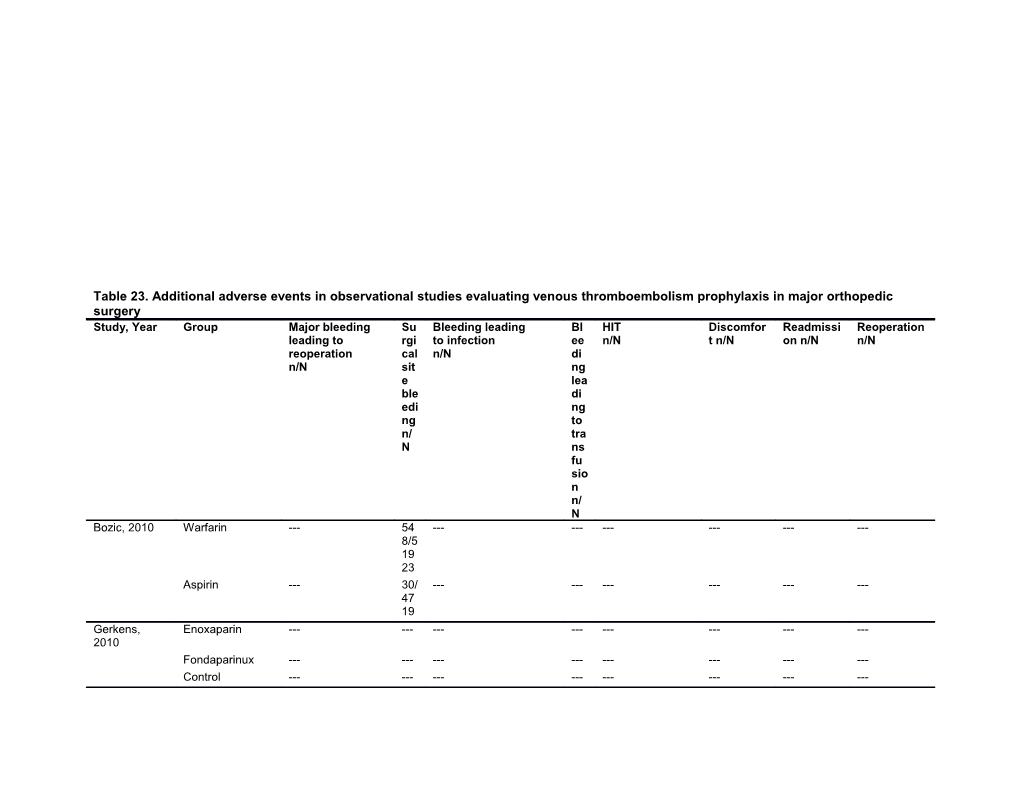
Table 23. Additional adverse events in observational studies evaluating venous thromboembolism prophylaxis in major orthopedic surgery Study, Year Group Major bleeding Su Bleeding leading Bl HIT Discomfor Readmissi Reoperation leading to rgi to infection ee n/N t n/N on n/N n/N reoperation cal n/N di n/N sit ng e lea ble di edi ng ng to n/ tra N ns fu sio n n/ N Bozic, 2010 Warfarin --- 54 ------8/5 19 23 Aspirin --- 30/ ------47 19 Gerkens, Enoxaparin ------2010 Fondaparinux ------Control ------Study, Year Group Major bleeding Su Bleeding leading Bl HIT Discomfor Readmissi Reoperation leading to rgi to infection ee n/N t n/N on n/N n/N reoperation cal n/N di n/N sit ng e lea ble di edi ng ng to n/ tra N ns fu sio n n/ N Cusick, 2009 Aspirin ------THR Warfarin ------Control ------Cusick, 2009 Aspirin ------TKR Warfarin ------Control ------Froimson, IPC (ActiveCare) ------2009 IPC (Flowtron) ------Shorr, 2007 Fondaparinux ------Enoxaparin or ------dalteparin UFH ------Control ------Sachs, 2003 Warfarin ------1/9 ------17/957 11/957 57 Control ------0/7 ------7/785 2/785 85 Abbreviations: HIT=heparin-induced thrombocytopenia; IPC=intermittent pneumatic compression; THR=total hip replacement; TKR=total knee replacement; UFH=unfractionated heparin
Table 23. Additional Adverse Events in Observational Studies Evaluating Venous Thromboembolism
Total Page:16
File Type:pdf, Size:1020Kb
Recommended publications