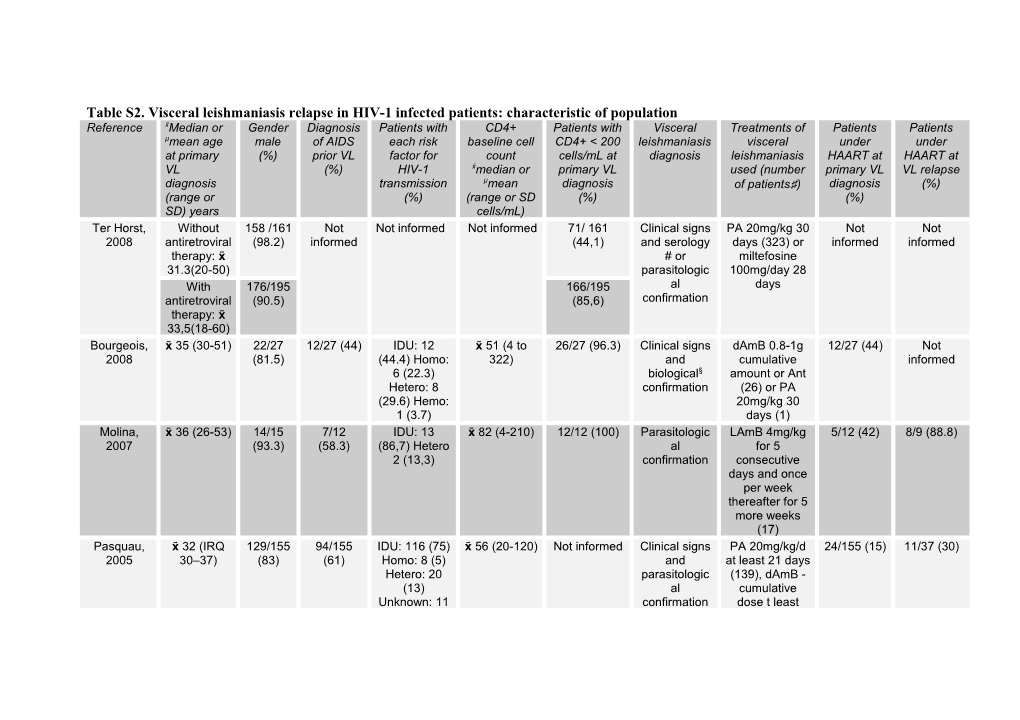
Table S2. Visceral leishmaniasis relapse in HIV-1 infected patients: characteristic of population Reference xMedianx or Gender Diagnosis Patients with CD4+ Patients with Visceral Treatments of Patients Patients μmean age male of AIDS each risk baseline cell CD4+ < 200 leishmaniasis visceral under under at primary (%) prior VL factor for count cells/mL at diagnosis leishmaniasis HAART at HAART at VL (%) HIV-1 xmedianx or primary VL used (number primary VL VL relapse diagnosis transmission μmean diagnosis of patients♯) diagnosis (%) (range or (%) (range or SD (%) (%) SD) years cells/mL) Ter Horst, Without 158 /161 Not Not informed Not informed 71/ 161 Clinical signs PA 20mg/kg 30 Not Not 2008 antiretroviral (98.2) informed (44,1) and serology days (323) or informed informed therapy: xx # or miltefosine 31.3(20-50) parasitologic 100mg/day 28 With 176/195 166/195 al days antiretroviral (90.5) (85,6) confirmation therapy: xx 33,5(18-60) Bourgeois, xx 35 (30-51) 22/27 12/27 (44) IDU: 12 xx 51 (4 to 26/27 (96.3) Clinical signs dAmB 0.8-1g 12/27 (44) Not 2008 (81.5) (44.4) Homo: 322) and cumulative informed 6 (22.3) biological§ amount or Ant Hetero: 8 confirmation (26) or PA (29.6) Hemo: 20mg/kg 30 1 (3.7) days (1) Molina, xx 36 (26-53) 14/15 7/12 IDU: 13 xx 82 (4-210) 12/12 (100) Parasitologic LAmB 4mg/kg 5/12 (42) 8/9 (88.8) 2007 (93.3) (58.3) (86,7) Hetero al for 5 2 (13,3) confirmation consecutive days and once per week thereafter for 5 more weeks (17) Pasquau, xx 32 (IRQ 129/155 94/155 IDU: 116 (75) xx 56 (20-120) Not informed Clinical signs PA 20mg/kg/d 24/155 (15) 11/37 (30) 2005 30–37) (83) (61) Homo: 8 (5) and at least 21 days Hetero: 20 parasitologic (139), dAmB - (13) al cumulative Unknown: 11 confirmation dose t least (7) 1.5g (7), lipid formulations of amphotericin - cumulative dose at least 1.5g (5), pentamidine 4mg/kg/d 2 weeks (1) or fluconazole + allopurinol (2) Mira, 2004 μ 33 (24-57) 19/21 8/10 (80) Not informed Not informed Not informed Clinical signs PA 0 19/21 (90) and 20mg/kg/day (90.5) μ 34 (26-37) 10/10 16/21 (76) parasitologic 28 days (13) or 0 10/10 (100) (100) al pentamidine confirmation 4mg/kg/day 28 days (2) or dAmB – 0.7mg/kg/d 28 days (5) or LAmB 2.5-4 mg/kg/d 10 days (9) or LipAmB 5mg/kg/day 14 days (5) López- μ 37+ 5 7/8 7/8 (87.5) Not informed Not informed Not informed Clinical signs Not informed Not 8/9 (88.9) Vélez, 2004 (87.5) and informed μ 5+ 6 9/9 4/9 (44.4) parasitologic 8/8 (100) (100) al confirmation Fernandéz- μ 34.6 31/34 Not IDU: 27 (77) Not informed Not informed Clinical signs Not informed 7/34 (20.6) 10/13 Cotarelo, (range: 27– (91.2) informed Homo: 4 and (76.9) 2003 60) (11,2) Hetero: parasitologic 2 (5.9) al Unknown: 1 confirmation (2.9) Bossolasco, xx 37 (30-42) 8/10 Not IDU: 7 (70). xx 42 (5-246) 9/10 (90) Clinical signs LAmB 4/10 (40) 5/7 (71.4) 2003 (80) informed Hetero: 2 (20) and 3mg/kg/day on Homo: 1 (10) parasitologic days 1-5 and al once weekly confirmation thereafter between 17-66 days (10) Casado, μ 34 (31-38) 6/10 Not IDU: 6 (60) xx 70 (3–156) 10/10 (100) Clinical signs PA 20mg/kg 28 0 10 (100) 2001 (60) informed Sexual: 4 (40) and days or dAmB parasitologic 0.7 mg/kg/day al 28 days confirmation Pizzuto, xx 32 (27-45) 8/10 8/10 (80) IDU: 8 (80) xx 70 (4-190) 10/10 (100) Clinical signs PA (4) or LAmB 5/10 (50) 7/10 (70) 2001 (80) Hetero: 2 (20) and serology (3) or dAmB (3) # or (“at standard parasitologic doses”) al confirmation Pintado, μ 33.2 + 8.2 64/80 43/80 IDU: 63 μ 90 (3–470) 61/70 (87.1) Clinical signs PA 20 2/73 (2.7) Not 2001 (80) (53.7) (78.7) Homo: and serology mg/kg/day - informed 6 (7.5) # or with a Hetero: 6 parasitologic maximum daily (7.5) al dose of 850 Perinatal: 1 confirmation mg) for 3–4 (1.3) weeks (51) or Unknown: 2 dAmB 0.5–1 (2.5) mg/day for 3–4 weeks (17) or Allopurinol + azoles compounds (4) Berenguer, xx 37 (24-47) 9/15 7/15 IDU: 9 (60) xx 77 (3-215) 13/14 (92.3) Clinical signs PA 20 mg/kg Not 15/15 (100) 2000 (60) (46.7) Homo: 2 and per day for 28 informed (13.3) Hetero: parasitologic days or LAmB 3 (20) al total of 10 Unknown: 1 confirmation doses of 4 (6.7) mg/kg per day given on days 1 to 5, 10, 17, 24, 31 and 38. Villanueva, μ 32.6 + 5,4 20/32 Not Not informed xx 50 (1-200) Not informed Clinical signs PA 20mg/kg/d Not 5/5 (100) 2000 (62.5) informed with HAART and 28 days (23) or informed xx 69 (27-166) parasitologic LipAmB without al 3mg/kg/d 5-10 HAART confirmation days (3) or LAmB 4mg/kg twice weekly 6 weeks (1) Laguna, xx 32 (19– 76/89 56/89 (63) IDU: 61 (69) xx 20 (0-231) Not informed Clinical signs PA 20 0 0 1999 64) (85) Sexual: 16 and mg/kg/day (44) (18) Others : parasitologic or dAmB - 0.7 12 (13) al mg/kg per day confirmation (45), both for 28 days Laguna, Not 42/43 29/43 (67) IDU: 35 (81) μ 10 ±3.3 Not informed Clinical signs PA low dose:< 0 0 1997 informed (98) Sexual: 7 and 20 mg/kg/day (16%) parasitologic 21 days (17) or Others :1 (2) al PA high dose: confirmation ≥20 mg at least 28 days (29) or dAmB (1) or LAmB (4) Fernandez, μ 34,3+ 5,1 30/31 18/31 IDU :21 μ 36,9 ± 27,9 31/31 (100) Clinical signs PA 20 mg/kg/d 0 0 1997 (96.7) (58.1) (67.7) Homo: and - maximum 6 (19.4) parasitologic 850mg/d 21 Hetero: 4 al days (21) or (12.9) confirmation dAmB 1-1.5 g cumulative amount - 0.5 mg/kg per day (20) Ribera, No Not 9/20 (45) IDU: 9 (45) xx 35 (2-125) Not informed Clinical signs PA 850mg/day 0 0 1996 prophylaxis informed and 21 days or xx 27 (20-64) parasitologic dAmB (52) Allopurinol 6/9 (67) IDU: 5 (55) xx 11 (2-42) al prophylaxis confirmation xx 28 (22-29) PA 3/17 (18) IDU: 13 (76) xx 34 (8-268) prophylaxis xx 29 (21-36) Montalban, Not 14/16 5/16 (31) IDU: 15 (94) Not informed Not informed Clinical signs PA (16) 0 0 1989 informed (87.5) Homo:1 (6) and parasitologic al confirmation
Table S2. Visceral Leishmaniasis Relapse in HIV-1 Infected Patients: Characteristic Of
Total Page:16
File Type:pdf, Size:1020Kb
Recommended publications