Point Defects in Lithium Gallate and Gallium Oxide
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

And Cobalt(III): Syntheses, Structures, and Ligand Field Parameters
Inorg. Chem. 2005, 44, 8459−8468 Homoleptic Trimethylsilylacetylide Complexes of Chromium(III), Iron(II), and Cobalt(III): Syntheses, Structures, and Ligand Field Parameters Louise A. Berben and Jeffrey R. Long* Contribution from the Department of Chemistry, UniVersity of California, Berkeley California 94720-1460 Received September 10, 2005 A straightforward method for synthesizing soluble homoleptic trimethylsilylacetylide complexes of first-row transition metal ions is presented. Reaction of anhydrous CrCl2 with an excess of LiCCSiMe3 in THF at −25 °C affords orange Li3[Cr(CCSiMe3)6]‚6THF (1), while analogous reactions employing M(CF3SO3)2 (M ) Fe or Co) generate pale yellow Li4[Fe(CCSiMe3)6]‚4LiCCSiMe3‚4Et2O(2) and colorless Li3[Co(CCSiMe3)6]‚6THF (3). Slightly modified reaction conditions lead to Li8[Cr2O4(CCSiMe3)6]‚6LiCCSiMe3‚4glyme (4), featuring a bis-µ-oxo-bridged binuclear complex, and Li3[Co(CCSiMe3)5(CCH)]‚LiCF3SO3‚8THF (5). The crystal structures of 1−3 show the trimethylsilyl- acetylide complexes to display an octahedral coordination geometry, with M−C distances of 2.077(3), 1.917(7)− 1.935(7), and 1.908(3) Å for M ) CrIII,FeII, and CoIII, respectively, and nearly linear M−CtC angles. The UV− 3- 4 4 visible absorption spectrum of [Cr(CCSiMe3)6] in hexanes exhibits one spin-allowed d−d transition ( T2g r A1g) 4- 3- and three lower-energy spin-forbidden d−d transitions. The spectra of [Fe(CCSiMe3)6] and [Co(CCSiMe3)6] in acetonitrile display high-intensity charge-transfer bands, which obscure all d−d transitions except for the lowest- 1 1 energy spin-allowed band ( T1g r A1g) of the latter complex. -

Z 221 PS2 Practice.Pages
CH 221 Practice Problem Set #2 This is a practice problem set and not the actual graded problem set that you will turn in for credit. Answers to each problem can be found at the end of this assignment. Covering: Chapter Two, Chapter 3.1 and Chapter Guide Two Important Tables and/or Constants: 1 mol = 6.022 x 1023 ! 1. Give the mass number of each of the following atoms: a. magnesium with 15 neutrons, b. titanium with 26 neutrons, and c. zinc with 32 neutrons. A 2. Give the complete symbol ( ZX) for each of the following atoms: a. potassium with 20 neutrons, b. krypton with 48 neutrons, and c. cobalt with 33 neutrons. 3. Thallium has two stable isotopes, 203Tl and 205Tl. Knowing that the atomic weight of thallium is 204.4, which isotope€ is the more abundant of the two? 4. Silver (Ag) has two stable isotopes, 107Ag and 109Ag. The isotopic mass of 107Ag is 106.9051 and the isotopic mass of 109Ag is 108.9047. The atomic weight of Ag, from the periodic table, is 107.868. Estimate the percentage of 107Ag in a sample of the element. a. 0% b. 25% c. 50% d. 75% 5. Gallium has two naturally occurring isotopes, 69Ga and 71Ga, with masses of 68.9257 u and 70.9249 u, respectively. Calculate the percent abundances of these isotopes of gallium. 6. Calculate the mass in grams of: a. 2.5 mol aluminum b. 1.25 x 10-3 mol of iron c. 0.015 mol of calcium d. -

Uncertainties in Cancer Risk Coefficients for Environmental Exposure to Radionuclides
OAK RIDGE ORNL/TM-2006/583 NATIONAL LABORATORY MANAGED BY UT-BATTELLE FOR THE DEPARTMENT OF ENERGY Uncertainties in Cancer Risk Coefficients for Environmental Exposure to Radionuclides An Uncertainty Analysis for Risk Coefficients Reported in Federal Guidance Report No. 13 January 2007 Prepared by D. J. Pawela R. W. Leggettb K. F. Eckermanb C. B. Nelsona aOffice of Radiation and Indoor Air U.S. Environmental Protection Agency Washington, DC 20460 bOak Ridge National Laboratory Oak Ridge, Tennessee 37831 DOCUMENT AVAILABILITY Reports produced after January 1, 1996, are generally available free via the U.S. Department of Energy (DOE) Information Bridge: Web site: http://www.osti.gov/bridge Reports produced before January 1, 1996, may be purchased by members of the public from the following source: National Technical Information Service 5285 Port Royal Road Springfield, VA 22161 Telephone: 703-605-6000 (1-800-553-6847) TDD: 703-487-4639 Fax: 703-605-6900 E-mail: [email protected] Web site: http://www.ntis.gov/support/ordernowabout.htm Reports are available to DOE employees, DOE contractors, Energy Technology Data Exchange (ETDE) representatives, and International Nuclear Information System (INIS) representatives from the following source: Office of Scientific and Technical Information P.O. Box 62 Oak Ridge, TN 37831 Telephone: 865-576-8401 Fax: 865-576-5728 E-mail: [email protected] Web site: http://www.osti.gov/contact.html This report was prepared as an account of work sponsored by an agency of the United States Government. Neither the United States government nor any agency thereof, nor any of their employees, makes any warranty, express or implied, or assumes any legal liability or responsibility for the accuracy, completeness, or usefulness of any information, apparatus, product, or process disclosed, or represents that its use would not infringe privately owned rights. -

Inorganic Chemistry , 6Th Ed
Chapter 1 8 Interpretation of Spectra In the previous chapter the topic of spectral studies on coordination compounds was introduced only briefl y in connection with ligand fi eld theory, and some of the attendant problems that are associ- ated with interpreting the spectra were described. In this chapter, a more complete description will be presented of the process of interpreting spectra of complexes. It is from the analysis of spectra that we obtain information about energies of spectroscopic states in metal ions and the effects produced by different ligands on the d orbitals. However, it is fi rst necessary to know what spectroscopic states are appropriate for various metal ions. The analysis then progresses to how the spectroscopic states for the metal ions are affected by the presence of the ligands and how ligand fi eld parameters are determined for spectral data. 18.1 SPLITTING OF SPECTROSCOPIC STATES As we have seen, an understanding of spin-orbit coupling is necessary to determine the spectroscopic states that exist for various electron confi gurations, d n (see Section 2.6). Because they will be needed frequently in this chapter, the spectroscopic states that result from spin-orbit coupling in d n ions that have degenerate d orbitals are summarized in Table 18.1 . The spectroscopic states shown in Table 18.1 are those that arise for the so-called free or gaseous ion. When a metal ion is surrounded by ligands in a coordination compound, those ligands generate an electrostatic fi eld that removes the degeneracy of the d orbitals. The result is that eg and t 2g subsets of orbitals are produced. -

Isotope Geochemistry of Gallium in Hydrothermal Systems
ISOTOPE GEOCHEMISTRY OF GALLIUM IN HYDROTHERMAL SYSTEMS by Constance E. Payne A thesis submitted to Victoria University of Wellington in partial fulfilment of the requirements for the degree of Master of Science Victoria University of Wellington 2016 ABSTRACT Little is known about the isotope geochemistry of gallium in natural systems (Groot, 2009), with most information being limited to very early studies of gallium isotopes in extra-terrestrial samples (Aston, 1935; De Laeter, 1972; Inghram et al., 1948; Machlan et al., 1986). This study is designed as a reconnaissance for gallium isotope geochemistry in hydrothermal systems of New Zealand. Gallium has two stable isotopes, 69Ga and 71Ga, and only one oxidation state, Ga3+, in aqueous media (Kloo et al., 2002). This means that fractionation of gallium isotopes should not be effected by redox reactions. Therefore the physical processes that occur during phase changes of hydrothermal fluids (i.e. flashing of fluids to vapour phase and residual liquid phase) and mineralisation of hydrothermal precipitates (i.e. precipitation and ligand exchange) can be followed by studying the isotopes of gallium. A gallium anomaly is known to be associated with some hydrothermal processes as shown by the unusual, elevated concentrations (e.g. 290 ppm in sulfide samples of Waiotapu; this study) in several of the active geothermal systems in New Zealand. The gallium isotope system has not yet been investigated since the revolution of high precision isotopic ratio measurements by Multi-Collector Inductively Coupled Plasma Mass Spectrometry (MC-ICPMS) and so a new analytical methodology needed to be established. Any isotopic analysis of multi-isotope elements must satisfy a number of requirements in order for results to be both reliable and meaningful. -

The Intensity of Ligand Absorption
Western Kentucky University TopSCHOLAR® Masters Theses & Specialist Projects Graduate School 8-1-1973 The nI tensity of Ligand Absorption Shing-Bong Chen Western Kentucky University Follow this and additional works at: http://digitalcommons.wku.edu/theses Part of the Chemistry Commons Recommended Citation Chen, Shing-Bong, "The nI tensity of Ligand Absorption" (1973). Masters Theses & Specialist Projects. Paper 1018. http://digitalcommons.wku.edu/theses/1018 This Thesis is brought to you for free and open access by TopSCHOLAR®. It has been accepted for inclusion in Masters Theses & Specialist Projects by an authorized administrator of TopSCHOLAR®. For more information, please contact [email protected]. ny THE INTENSITY OF LIGAND ABSORPTION A Thesis Presented to the Faculty of the Department of Chemistry Western Kentucky University Bowling Green, Kentucky in Partial Fulfillment of the Requirements for the Degree Master of Science by Shing-Bong Chen August 1973 THE INTENSITY OP LIGAND ABSORPTION APPROVED (Date) Director of ' TheSIS —— r •Mb >„ fir t-73 :an of the Graduate School ACKNOWLEDGMENT I would like to express gratitude to my research advisor, Dr. Earl F. Pearson, who has given freely of his time and knowledge to show me the way to scientific research. His critical advice and encour- agement are especially appreciated. In addition, I wish to thank the members of the Department of Chemistry of VJestern Kentucky University for their invaluable discussions and suggestions. I also wish to thank Mrs. Linda Moore for typing this thesis. Shing-Bong Chen iii TABLE OP CONTENTS Page ACKNOVJIEDGEJ-'ENTS ±±± LIST OF TABLES v LIST OF FIGURES ; vl ABSTRACT vll INTRDDUCTICN 1 Chapter I. -
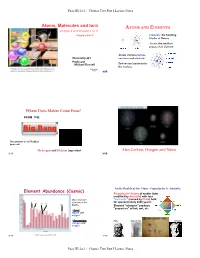
ATOMS and ELEMENTS Chapter 2 and Chapter 3 (3.1) “Chapter 2 Part 1” Elements: the Building Blocks of Nature Atoms: the Smallest Pieces of an Element
Page III-2a-1 / Chapter Two Part I Lecture Notes Atoms, Molecules and Ions ATOMS AND ELEMENTS Chapter 2 and Chapter 3 (3.1) “Chapter 2 Part 1” Elements: the building blocks of Nature Atoms: the smallest pieces of an element Atoms contain protons, Chemistry 221 neutrons and electrons Professor Michael Russell Protons and neutrons in the nucleus Last update: MAR 8/9/21 MAR Where Does Matter Come From? FROM THE Big Bang The universe is 13.77 billion years old Hydrogen and Helium important Also Carbon, Oxygen and Neon MAR MAR Early Models of the Atom - Empedocles & Aristotle Element Abundance (Cosmic) Empedocles’ theory of matter (later modified by Aristotle) with four Most abundant "elements" (coined by Plato) held C elements in the for approximately 2000 years! O Al Si Fe Earth's Element "mixtures" produce "properties" of hot, wet, etc. • Crust: Silicon and oxygen • Atmosphere: Plato Empedocles Aristotle nitrogen and oxygen MAR http://www.webelements.com/ MAR Page III-2a-1 / Chapter Two Part I Lecture Notes Page III-2a-2 / Chapter Two Part I Lecture Notes The "Newton" of Chemistry Early Models of the Atom - Democritus JOHN DALTON (1766 - 1844) DEMOCRITUS (460 - 370 BCE) 1804 - Proposed Atomic Theory was a contemporary of Plato "Atoms cannot be created or destroyed" "Atoms of one element are different from other Atoms have structure and volume element's atoms" - proposed relative scale of "Gold can be divided into atomic masses (now the amu) smaller pieces only so far before the pieces no longer "Chemical change involves bond breaking, retain -

Rapid Colorimetric Detection of Cyanide
Zurich Open Repository and Archive University of Zurich Main Library Strickhofstrasse 39 CH-8057 Zurich www.zora.uzh.ch Year: 2012 Rapid Colorimetric Detection of Cyanide Männel-Croisé, Christine Posted at the Zurich Open Repository and Archive, University of Zurich ZORA URL: https://doi.org/10.5167/uzh-74766 Dissertation Published Version Originally published at: Männel-Croisé, Christine. Rapid Colorimetric Detection of Cyanide. 2012, University of Zurich, Faculty of Science. Rapid Colorimetric Detection of Cyanide Dissertation zur Erlangung der naturwissenschaftlichen Doktorwürde (Dr. sc. nat.) vorgelegt der Mathematisch-naturwissenschaftlichen Fakultät der Universität Zürich von Christine Männel-Croisé aus Deutschland Promotionskomitee Prof. Dr. Roger Alberto (Vorsitz) Dr. Felix Zelder (Leitung) Prof. Dr. Roland K. O. Sigel Zürich 2012 Summary iii New strategies for the rapid, straightforward colorimetric detection of cyanide in complex samples with corrin-based chemosensors have been developed. In principle, laboratory equipment is not required. The methods are, therefore, promising for applications by non-expert users for the detection of blood cyanide in emergency situations and for water and food analysis, particularly in tropical countries. Challenges in these situations are posed by the complex sample medium, the low levels of detection and the selectivity of the methods. This thesis presents the first method for the rapid visual detection of blood cyanide using immobilised corrinoids and solid-phase extraction. The result in form of a colour change is obtained within a few minutes using only 1 mL of blood. The semi- quantitative determination of blood cyanide content is achieved by means of a colour chart whereas quantitative determinations are possible by diffuse reflectance spectroscopy or a hand-held spectrophotometer. -

Laser Spectroscopy of Gallium Isotopes Using the ISCOOL RFQ
Addendum to INTC-P-224 / IS457 Laser spectroscopy of gallium isotopes using the ISCOOL RFQ cooler. J. Billowes1, M.L. Bissell2, K. Blaum 3, B. Cheal1, K.T. Flanagan1, D.H. Forest4, C. Geppert5, M. Kowalska6, K. Kreim3, I.D. Moore7, R. Neugart8, G. Neyens2, W. N¨ortersh¨auser8, H.H. Stroke9, G. Tungate4, D.T. Yordanov6 1 Schuster Bldg, The University of Manchester, Brunswick Street, Manchester, M13 9PL, UK 2 Instituut voor Kern- en Stralingsfysica, KU Leuven, B-3001 Leuven, Belgium 3 Max-Planck-Institut f¨urKernphysik, D-69117 Heidelberg, Germany 4 School of Physics and Astronomy, The University of Birmingham, Birmingham, B15 2TT, UK 5 GSI Helmholtzzentrum f¨urSchwerionenforschung GmbH, D-64291 Darmstadt, Germany 6 Physics Department, CERN, CH-1211 Geneva 23, Switzerland 7 Department of Physics, University of Jyv¨askyl¨a,PB 35 (YFL) FIN-40014 Jyv¨askyl¨a,Finland 8 Institut f¨urKernchemie, Universit¨atMainz, D-55128 Mainz, Germany 9 Department of Physics, New York University, 4 Washington Place, New York, NY 10003, USA Spokesperson: Bradley Cheal Contact Person: Mark Bissell At the February 2007 meeting of the INTC, the above proposal was submitted to per- form laser spectroscopy of both neutron-rich and neutron-deficient isotopes of gallium using the newly installed ISCOOL. The INTC awarded 15 shifts for the study of the neutron-rich isotopes and requested that a status report then be submitted|discussing the measurements, performance of ISCOOL and beam purity|before proceeding to the neutron-deficient cases. The report is given here, with a request for 9 shifts to continue the study to the neutron-deficient isotopes. -

Advanced Inorganic Chemistry ADVANCED INORGANIC CHEMISTRY Diagrams Orgel a Splitting of the Weak Field Dn Ground State Terms in an Octahedral Ligand Field DVANCED
Advanced Inorganic Chemistry ADVANCED INORGANIC CHEMISTRY Diagrams Orgel A Splitting of the weak field dn ground state terms in an octahedral ligand field DVANCED Correlation of spectroscopic terms for dn configuration in O complexes I h NORGANIC om c um er Terms in O At i N b h Term of states Symmetry S 1 A1g P 3 T 1g D 5 T2g + Eg C HEMISTRY F 7 T1g + T2g + A2g Ground state determined by inspection of degeneracy of terms for given dn ADVANCED INORGANIC CHEMISTRY A Orgel Diagrams DVANCED 3 T1g(P) 4 T1g(P) I 3P 3A 4P NORGANIC 2 2g 5 Eg 4 T2g T1g 3 T2g 2 3 4 4 5 D F F T2g D C 2 3 5 HEMISTRY T2g T1g Eg 1 3 ∆o 4 ∆o 2 ∆ d 4 d ∆ d d o A2g o 2 2 T2g E 3 3 g T1g T2g 3 3 T1g A2g 3 3 T1g T1g(P) Ti3+ V2+ Cr3+ Mn3+ A DVANCED The d-d bands of the d2 ion [V(H O) ]3+ 2 6 I NORGANIC C HEMISTRY The Tanabe-Sugano diagram ADVANCED INORGANIC CHEMISTRY A DVANCED Correlation diagrams between energies of atomic and molecular terms can drawn as so-called I NORGANIC Tanabe-Sugano diagrams for each electron configuration of free ions. C HEMISTRY Y. Tanabe, s. Sugano; J. Phys. Soc, Jap., 9, 753 (1954) Energy values in a Tanabe-Sugano diagram are only given relative to the ground state (x-axis). A DVANCED The simple correlation diagram had multiples of B (the Racah parameter) on the energy axis to denote the relative energies of the atomic terms. -

Development of Antibody Immuno-PET/SPECT Radiopharmaceuticals for Imaging of Oncological Disorders—An Update
cancers Review Development of Antibody Immuno-PET/SPECT Radiopharmaceuticals for Imaging of Oncological Disorders—An Update Jonatan Dewulf 1,3 , Karuna Adhikari 2, Christel Vangestel 1,3 , Tim Van Den Wyngaert 1,3 and Filipe Elvas 1,3,* 1 Molecular Imaging Center Antwerp, Faculty of Medicine and Health Sciences, University of Antwerp, Universiteitsplein 1, B-2610 Wilrijk, Belgium; [email protected] (J.D.); [email protected] (C.V.); [email protected] (T.V.D.W.) 2 Faculty of Pharmaceutical Biomedical and Veterinary Sciences, Medicinal Chemistry, University of Antwerp, Universiteitsplein 1, B-2610 Wilrijk, Belgium; [email protected] 3 Department of Nuclear Medicine, Antwerp University Hospital, Wilrijkstraat 10, B-2650 Edegem, Belgium * Correspondence: fi[email protected] Received: 8 June 2020; Accepted: 10 July 2020; Published: 11 July 2020 Abstract: Positron emission tomography (PET) and single-photon emission computed tomography (SPECT) are molecular imaging strategies that typically use radioactively labeled ligands to selectively visualize molecular targets. The nanomolar sensitivity of PET and SPECT combined with the high specificity and affinity of monoclonal antibodies have shown great potential in oncology imaging. Over the past decades a wide range of radio-isotopes have been developed into immuno-SPECT/PET imaging agents, made possible by novel conjugation strategies (e.g., site-specific labeling, click chemistry) and optimization and development of novel radiochemistry procedures. In addition, new strategies such as pretargeting and the use of antibody fragments have entered the field of immuno-PET/SPECT expanding the range of imaging applications. Non-invasive imaging techniques revealing tumor antigen biodistribution, expression and heterogeneity have the potential to contribute to disease diagnosis, therapy selection, patient stratification and therapy response prediction achieving personalized treatments for each patient and therefore assisting in clinical decision making. -

Exam I Answers
CHEM 1314 3;30 pm Theory Name ________________________ Exam !I John I. Gelder TA's Name ________________________ September 11, 2002 Lab Section ______________________ INSTRUCTIONS: 1. This examination consists of a total of 6 different pages. The last page include a periodic table and some useful equations. All work should be done in this booklet. 2. PRINT your name, TA's name and your lab section number now in the space at the top of this sheet. DO NOT SEPARATE THESE PAGES. 3. Answer all questions that you can and whenever called for show your work clearly. Your method of solving problems should pattern the approach used in lecture. 4. No credit will be awarded if your work is not shown in problems 2, 4 and 7a. 5. Point values are shown next to the problem number. 6. Budget your time for each of the questions. Some problems may have a low point value yet be very challenging. If you do not recognize the solution to a question quickly, skip it, and return to the question after completing the easier problems. 7. Look through the exam before beginning; plan your work; then begin. 8. Relax and do well. Page 2 Page 2 Page 3 Page 4 Page 5 TOTAL SCORES _____ _____ _____ _____ _____ ______ (1) (30) (24) (29) (16) (100) CHEM 1314 EXAM I PAGE 2 (12) 1. Write the chemical formula(s) of the product(s) and balance all of the following reactions. Identify all products phases as either (g)as, (l)iquid, (s)olid or (aq)ueous a) 3Mg(s) + N2(g) Æ Mg3N2(s) b) S8(g) + 8O2(g) Æ 8SO2(g) c) 2C6H14(l) + 19O2(g) Æ 12CO2(g) + 14H2O(g) d) 2K(s) + Cl2(g) Æ 2KCl(s) Grading: 3 points for the correct products, balance and phases.