Problem Set #10 Suggested Answers to Problems 11.1, 11.3, 11.5, 11.6 and 11.7
11.1 Digging clams by hand in Sunset Bay requires only labor input. The total number of clams obtained per hour (q) is given by
q = 100L where L is labor input per hour.
a. Graph the relationship between q and L.
300
250 TPL
200
150
100
50
0 0 2 4 6 8
b. What is the average productivity of labor in Sunset Bay? Graph this relationship and show that APL diminishes for increases in labor input.
APL = 100/L. APL moves inversely with L See graph below.
c. Show that the marginal productivity of labor in Sunset Bay is given by
MPL = 50/L
dq/dL = .5(100)L-.5
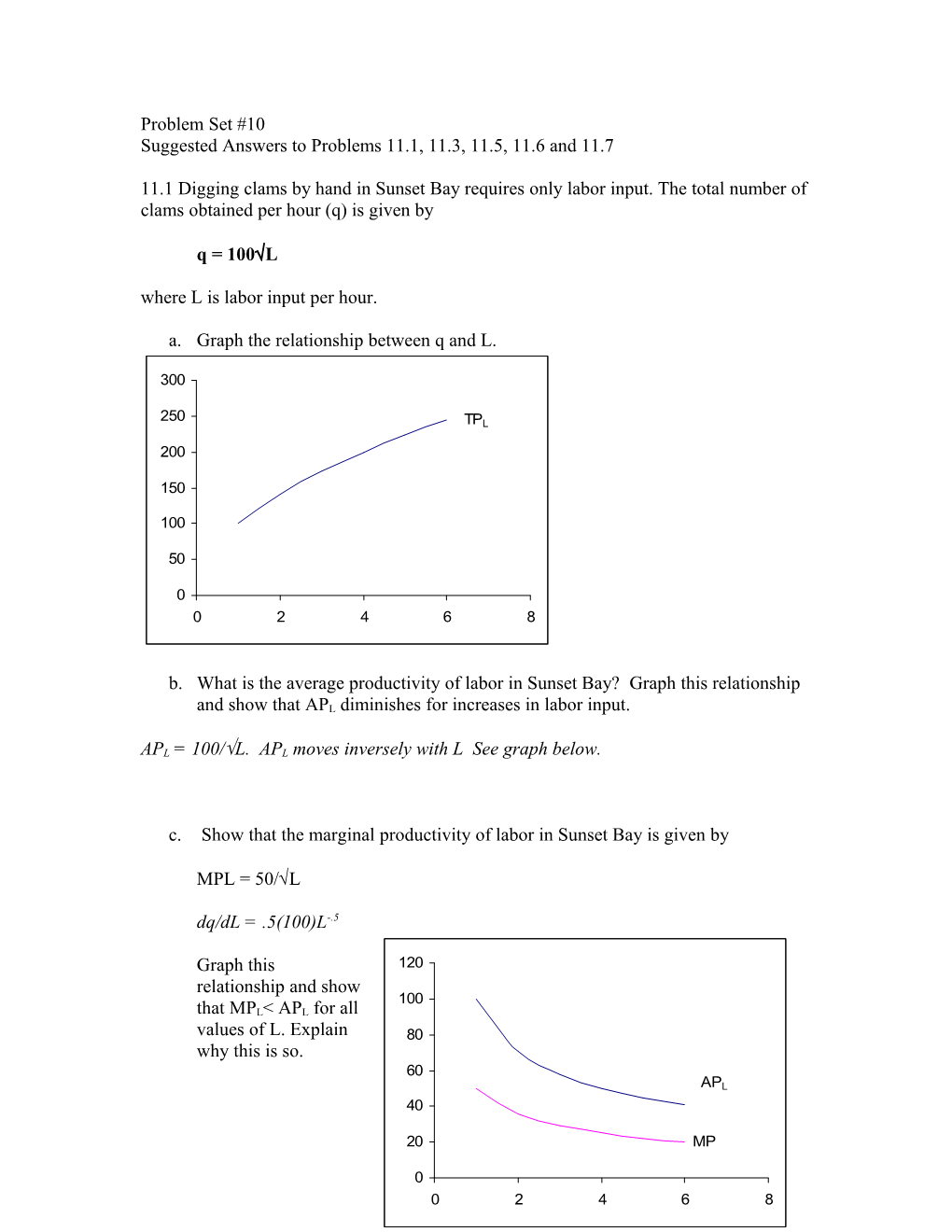
Graph this 120 relationship and show 100 that MPL< APL for all values of L. Explain 80 why this is so. 60 APL 40
20 MP 1 L 0 0 2 4 6 8 See graph on the left. The Marginal “drives” the average. When the marginal is below the average it pulls it down.
11.3. Power Goat Lawn Company uses two sizes of mowers to cut lawns. The smaller mowers have a 24-inch blade and are used on lawns with many trees and obstacles. The larger mowers are exactly twice as big as the smaller mowers and are used on open lawns where maneuverability is not so difficult. The two production functions available to Power Goat are:
Output per hour Capital Input Labor Input (Square Feet) (# of 24” mowers) Large Mowers 8000 2 1 Small Mowers 5000 1 1
a. Graph the q= 40,000 square feet isoquant for the first production function. How
K 16 14 12 10 q=40,000K 8 6 4 2 0 0 2 4 6 8 10 L
much K and L would be used if these factors were combined without waste? Q=40,000 with 5*2 = 10 units of capital and 5*1 =5 units of labor. However, the production technology allows no substitutability. Thus, the production function is lexographical.
b. Answer part (a) for the second function.
2 For q=40,000, K K 16 =8 and L = 8. 14 See graph on 12 right. 10 q=40,000K
8 6 4 2 0 0 2 4 6 8 10 12 14 16 L
c. How much K and L would be used without waste if half of the 40,000-square-foot lawn were cut by the method of the first production function and half by the method of the second? How much K and L would be used if three-fourths of the lawn were cut by the first method and one-fourth by the second? What does it mean to speak of fractions of K and L?
40,000 sq. ft. LM SM LM/SM mix L K L K 0/100$% 0 0 8 8 25%/75% 1.25 2.5 6 6 50%/50% 2.5 5 4 4 75%/25% 3.75 7.5 2 2 100%/0 5 10 0 0 Fractions of K and L are fractions of an hour.
d. On the basis of your observations in part (c), draw a q=40,000 isoquant for the combined production functions.
40,000 sq. ft. LM/SM mix L K 0/100$% 8 8 25%/75% 7.25 8.5 50%/50% 6.5 9 75%/25% 5.75 9.5 100%/0 5 10
3 13
12
11
10 q=40,000 9
8
7
6
5 0 5 10 15
Observe that even when two technologies use fixed inputs, if combinations of those technologies can be made, substitutability between the primary inputs be made.
4 11.5 Suppose that q = L K 0<<1, 0<<1, +=1.
a. Show that eq,L = , eq,K =
eq,L = (q/L)(L/q) = (L-1K)(L/ L-1K)
=
eq,K = (q/K)(K/q) = (LK-1)(K/ LK-1)
=
2 2 2 2 b. Show that MPL>0, MPK>0, q/L <0 , q/K <0
-1 MPL = (q/L) = L K For L, K >0 this is positive.
-1 MPK = (q/K) = L K For L, K >0 this is positive.
2 2 -2 q/L = (MPL/L) = (-1)L K Since <1, this is negative
2 2 -2 q/K =(MPK/K) = (-1)L K Since <1, this is negative c. Show that the RTS depends only on K/L, but not on the scale of production, and that the RTS (L for K) diminishes as L/K increases
-1 -1 RTS (L for K) = MPL/MPK = (L K )/L K = (K)/(L)
This is a CRS function. That is, any increase in all inputs a will increase output by a
(aL)(aK) = a+LK = a LK
Increasing the scale of production will not affect the RTS, because the scale term “a” cancels out in the RTS expression.
Further L/K enters the RTS inversely, so L/K increases implies RTS falls
5 11.6. Show that for the constant returns-to-scale CES production function 1/ q = [K + L ]
1- 1- a. MPK = (q/K) and that MPL = (q/L)
(1-)/ -1 MPL = (q/L) =(/)[K + L ] L = (q/L) (1-)
(1-)/ -1 MPK = (q/K) =(/)[K + L ] K = (q/K) (1-) b. RTS(L for K) = (K/L) 1-. Use this to show that = 1/(1-)
(1-) (1-) RTS = MPL/MPK = [(q/L) ]/[(q/K) ] = [K/L](1-)
Observe that ln(RTS) = ln [K/L] d (lnRTS)/ d( ln K/L) = (1-)
If the implicit function theorem holds (and the relationship is invertible, d( ln K/L) /d (lnRTS) = 1/(1-) = c. Determine the output elasticities for K and L. Show that their sum equals 1.
(1-) eq,L = (q/L)[L/q] = MPL[L/q] =(q/L) [L/q] = L/q = L /[K+ L] Similarly
(1-) eq,K = (q/K)[K/q] = MPK[K/q] =(q/K) [K/q] = K/q = K /[K+ L] d. Prove that (q/L) = (q/L) Hence show ln (q/L) = ln (q/L)
q/L = (q/L) (1-) = (L/q) Since = 1/(1-)
Taking logarithms ln(q/L) = ln (q/L)
6 11.7 Consider a production function of the form ½ q = o + 1 (KL) + 2 K+ 3 L where
0< i<1 i = 0, …, 3. a. If this function is to exhibit constant returns to scale, what restrictions should be placed on the parameters o, … 3?
CRS implies that this function is homogeneous of degree 1. Thus, we need for
½ mq = o + 1 (mKmL) + 2 mK+ 3 mL
This can only hold if o =0. No other restrictions are necessary. b. Show that in the constant-returns-to-scale case this function exhibits diminishing marginal productivities and that the marginal productivity functions are homogeneous of degree zero.
½ ½ q/L = ½ 1 (K /L ) +3>0 ½ 3/2 MPL/L= - ¼ 1((K /L )<0
Similarly ½ ½ q/K = ½ 1 (L /K ) +2 >0 ½ 3/2 MPK/K= - ¼ 1((K /L ) <0
The degree-zero homogeneity of the marginal productivity functions is obvious, since K and L are reciprocally related in each case. c. Calculate in this case. Is constant?
= [d(K/L)/dRTS][RTS/(K/L)}
Observe that the RTS is
½ ½ ½ ½ RTS = MPL/MPK = [½ 1 (K /L ) +3]/[ ½ 1 (L /K ) +2]
Sorry, but this isn’t pretty! It is obvious from the RTS that we cannot develop an expression for RTS that will allow for application of the implicit function theorem. By brute force, I managed to get an expression for that is
1/2 = ¼ 1 (K/L) ( 3 - 2)
¼ (K/L) + ½ 1(K/L)( 2+3) +23
If correct, this is clearly not a constant. This is what you would expect, given the linearly additive terms in the RTS.
7 8