The Factors Affecting Productivity and Parental
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

First Bolivian Record of Laughing Gull Leucophaeus Atricilla, and Two Noteworthy Records of Fulica Coots from Laguna Guapilo, Dpto
Cotinga 41 First Bolivian record of Laughing Gull Leucophaeus atricilla, and two noteworthy records of Fulica coots from Laguna Guapilo, dpto. Santa Cruz Matthew L. Brady, Anna E. Hiller, Damián I. Rumiz, Nanuq L. Herzog-Hamel and Sebastian K. Herzog Received 30 November 2018; fnal revision accepted 29 April 2019 Cotinga 41 (2019): 98–100 published online 21 June 2019 El 28 de enero de 2018, durante una visita a laguna Guapilo, al este de Santa Cruz de la Sierra, depto. Santa Cruz, Bolivia, observamos una Gaviota Reidora Leucophaeus atricilla, el primer registro en Bolivia. Adicionalmente, observamos comportamiento indicativo de anidación de la Gallareta Chica Fulica leucoptera, una especie que se consideraba como visitante no reproductiva en Bolivia, así como una Gallareta Andina Fulica ardesiaca, el primer registro para el depto. Santa Cruz. La reproducción de F. leucoptera en la laguna Guapilo fue confrmada el 5 de mayo de 2018 mediante la fotografía de un polluelo. On 28 January 2018, MLB, AEH, NLH-H and We aged the bird during the observation SKH observed several notable birds at Laguna based on the following combination of characters: Guapilo (17°46’50”S 63°05’48”W), a semi-urban uniformly dark primaries, without the white apical park 8.9 km east of Santa Cruz city centre, dpto. spots typical of older birds; a dark tail-band; Santa Cruz, Bolivia. The habitat is dominated by a extensive ash-grey neck and breast; and worn, c.35-ha lagoon, with dense mats of reeds and water brownish wing-coverts. These features are typical hyacinth Eichhornia crassipes at the edges, and of an advanced frst-year L. -

American Oystercatcher
Maine 2015 Wildlife Action Plan Revision Report Date: January 13, 2016 Haematopus palliatus (American Oystercatcher) Priority 3 Species of Greatest Conservation Need (SGCN) Class: Aves (Birds) Order: Charadriiformes (Plovers, Sandpipers, And Allies) Family: Haematopodidae (Oystercatchers) General comments: First nesting record in Maine - 1994. 4 - 8 breeding pairs in Maine, range expansion, Northern Atlantic population 11,000 and considered stable. Listed as Critical risk category because of high vulnerability to climate change - Gilbrathe et al. 2014. Species Conservation Range Maps for American Oystercatcher: Town Map: Haematopus palliatus_Towns.pdf Subwatershed Map: Haematopus palliatus_HUC12.pdf SGCN Priority Ranking - Designation Criteria: Risk of Extirpation: NA State Special Concern or NMFS Species of Concern: Haematopus palliatus is listed as a species of Special Concern in Maine. Recent Significant Declines: NA Regional Endemic: NA High Regional Conservation Priority: United States Shorebird Conservation Plan: Species of High Concern North Atlantic Regional Shorebird Plan: Highly Imperiled United States Birds of Conservation Concern: Bird of Conservation Concern in Bird Conservation Regions 14 and/or 30: Yes High Climate Change Vulnerability: Vulnerability: 3, Confidence: High, Reviewers: Decided in Workshop (W) Understudied rare taxa: NA Historical: NA Culturally Significant: NA Habitats Assigned to American Oystercatcher: Formation Name Cliff & Rock Macrogroup Name Rocky Coast Formation Name Intertidal Macrogroup Name Intertidal -

Order CHARADRIIFORMES: Waders, Gulls and Terns Suborder LARI
Text extracted from Gill B.J.; Bell, B.D.; Chambers, G.K.; Medway, D.G.; Palma, R.L.; Scofield, R.P.; Tennyson, A.J.D.; Worthy, T.H. 2010. Checklist of the birds of New Zealand, Norfolk and Macquarie Islands, and the Ross Dependency, Antarctica. 4th edition. Wellington, Te Papa Press and Ornithological Society of New Zealand. Pages 191, 223 & 227-228. Order CHARADRIIFORMES: Waders, Gulls and Terns The family sequence of Christidis & Boles (1994), who adopted that of Sibley et al. (1988) and Sibley & Monroe (1990), is followed here. Suborder LARI: Skuas, Gulls, Terns and Skimmers Condon (1975) and Checklist Committee (1990) recognised three subfamilies within the Laridae (Larinae, Sterninae and Megalopterinae) but this division has not been widely adopted. We follow Gochfeld & Burger (1996) in recognising gulls in one family (Laridae) and terns and noddies in another (Sternidae). The sequence of species for Stercorariidae and Laridae follows Peters (1934) and for Sternidae follows Bridge et al. (2005). Family LARIDAE Rafinesque: Gulls Laridia Rafinesque, 1815: Analyse de la Nature: 72 – Type genus Larus Linnaeus, 1758. Genus Larus Linnaeus Larus Linnaeus, 1758: Syst. Nat., 10th edition 1: 136 – Type species (by subsequent designation) Larus marinus Linnaeus. Gavia Boie, 1822: Isis von Oken, Heft 10: col. 563 – Type species (by subsequent designation) Larus ridibundus Linnaeus. Junior homonym of Gavia Moehring, 1758. Hydrocoleus Kaup, 1829: Skizz. Entw.-Gesch. Eur. Thierw.: 113 – Type species (by subsequent designation) Larus minutus Linnaeus. Chroicocephalus Eyton, 1836: Cat. Brit. Birds: 53 – Type species (by monotypy) Larus cucullatus Reichenbach = Larus pipixcan Wagler. Gelastes Bonaparte, 1853: Journ. für Ornith. -

Morphological Variation Among Herring Gulls (Larus Argentatus) and Great Black-Backed Gulls (Larus Marinus) in Eastern North America Gregory J
View metadata, citation and similar papers at core.ac.uk brought to you by CORE provided by University of New England University of New England DUNE: DigitalUNE Environmental Studies Faculty Publications Environmental Studies Department 4-2016 Morphological Variation Among Herring Gulls (Larus Argentatus) And Great Black-Backed Gulls (Larus Marinus) In Eastern North America Gregory J. Robertson Environment Canada Sheena Roul Environment Canada Karel A. Allard Environment Canada Cynthia Pekarik Environment Canada Raphael A. Lavoie Queen's University See next page for additional authors Follow this and additional works at: http://dune.une.edu/env_facpubs Part of the Ornithology Commons Recommended Citation Robertson, Gregory J.; Roul, Sheena; Allard, Karel A.; Pekarik, Cynthia; Lavoie, Raphael A.; Ellis, Julie C.; Perlut, Noah G.; Diamond, Antony W.; Benjamin, Nikki; Ronconi, Robert A.; Gilliland, Scott .;G and Veitch, Brian G., "Morphological Variation Among Herring Gulls (Larus Argentatus) And Great Black-Backed Gulls (Larus Marinus) In Eastern North America" (2016). Environmental Studies Faculty Publications. 22. http://dune.une.edu/env_facpubs/22 This Article is brought to you for free and open access by the Environmental Studies Department at DUNE: DigitalUNE. It has been accepted for inclusion in Environmental Studies Faculty Publications by an authorized administrator of DUNE: DigitalUNE. For more information, please contact [email protected]. Authors Gregory J. Robertson, Sheena Roul, Karel A. Allard, Cynthia Pekarik, Raphael A. Lavoie, Julie C. Ellis, Noah G. Perlut, Antony W. Diamond, Nikki Benjamin, Robert A. Ronconi, Scott .G Gilliland, and Brian G. Veitch This article is available at DUNE: DigitalUNE: http://dune.une.edu/env_facpubs/22 Morphological Variation Among Herring Gulls (Larus argentatus) and Great Black-Backed Gulls (Larus marinus) in Eastern North America Author(s): Gregory J. -

Scottish Birds 22: 9-19
Scottish Birds THE JOURNAL OF THE SOC Vol 22 No 1 June 2001 Roof and ground nesting Eurasian Oystercatchers in Aberdeen The contrasting status of Ring Ouzels in 2 areas of upper Deeside The distribution of Crested Tits in Scotland during the 1990s Western Capercaillie captures in snares Amendments to the Scottish List Scottish List: species and subspecies Breeding biology of Ring Ouzels in Glen Esk Scottish Birds The Journal of the Scottish Ornithologists' Club Editor: Dr S da Prato Assisted by: Dr I Bainbridge, Professor D Jenkins, Dr M Marquiss, Dr J B Nelson, and R Swann Business Editor: The Secretary sac, 21 Regent Terrace Edinburgh EH7 5BT (tel 0131-5566042, fax 0131 5589947, email [email protected]). Scottish Birds, the official journal of the Scottish Ornithologists' Club, publishes original material relating to ornithology in Scotland. Papers and notes should be sent to The Editor, Scottish Birds, 21 Regent Terrace, Edinburgh EH7 SBT. Two issues of Scottish Birds are published each year, in June and in December. Scottish Birds is issued free to members of the Scottish Ornithologists' Club, who also receive the quarterly newsletter Scottish Bird News, the annual Scottish Bird Report and the annual Raplor round up. These are available to Institutions at a subscription rate (1997) of £36. The Scottish Ornithologists' Club was formed in 1936 to encourage all aspects of ornithology in Scotland. It has local branches which meet in Aberdeen, Ayr, the Borders, Dumfries, Dundee, Edinburgh, Glasgow, Inverness, New Galloway, Orkney, St Andrews, Stirling, Stranraer and Thurso, each with its own programme of field meetings and winter lectures. -

Status and Distribution of the Laridae in Wyoming Through 1986
Great Basin Naturalist Volume 54 Number 4 Article 6 10-25-1994 Status and distribution of the Laridae in Wyoming through 1986 Scott L. Findholt Wyoming Game and Fish Department, Lander, Wyoming Follow this and additional works at: https://scholarsarchive.byu.edu/gbn Recommended Citation Findholt, Scott L. (1994) "Status and distribution of the Laridae in Wyoming through 1986," Great Basin Naturalist: Vol. 54 : No. 4 , Article 6. Available at: https://scholarsarchive.byu.edu/gbn/vol54/iss4/6 This Article is brought to you for free and open access by the Western North American Naturalist Publications at BYU ScholarsArchive. It has been accepted for inclusion in Great Basin Naturalist by an authorized editor of BYU ScholarsArchive. For more information, please contact [email protected], [email protected]. Creat Basin Naturalist 54(4), e 1994, pp. 34:2:-350 STATUS AD DISTRIBUTIO OF THE LARIDAE IN WYOMI 'G THROUGH 1986 Scott L. Findhoh1 A5~TR.-\n.-To date, 17 species of Laridae have been reported in Wyoming. Six of these species have known breed· ing populations in the state; the Rjng~hilled Gull (Lams delalkuren.sis), Califomia Gull (Larm colifamicus), Herring Gull (Lams argentatus), Caspian Tern {Sterna caspia), Forster's Tern (Stemaforsteri), and Black Tern (Chlidonias niger). Of these species, the California Cull is the most abundant and widespread. In 1984 approximately 1300 nests existed in \Vyoming at six breeding locations consisting of 10 different colonies. In contrast, only smllll breeding populations have been discovered for the remaining five species. The Herring Gull is the most recent addition among Laridae known to nest in Wyoming. -
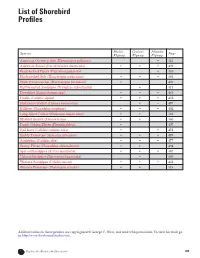
List of Shorebird Profiles
List of Shorebird Profiles Pacific Central Atlantic Species Page Flyway Flyway Flyway American Oystercatcher (Haematopus palliatus) •513 American Avocet (Recurvirostra americana) •••499 Black-bellied Plover (Pluvialis squatarola) •488 Black-necked Stilt (Himantopus mexicanus) •••501 Black Oystercatcher (Haematopus bachmani)•490 Buff-breasted Sandpiper (Tryngites subruficollis) •511 Dowitcher (Limnodromus spp.)•••485 Dunlin (Calidris alpina)•••483 Hudsonian Godwit (Limosa haemestica)••475 Killdeer (Charadrius vociferus)•••492 Long-billed Curlew (Numenius americanus) ••503 Marbled Godwit (Limosa fedoa)••505 Pacific Golden-Plover (Pluvialis fulva) •497 Red Knot (Calidris canutus rufa)••473 Ruddy Turnstone (Arenaria interpres)•••479 Sanderling (Calidris alba)•••477 Snowy Plover (Charadrius alexandrinus)••494 Spotted Sandpiper (Actitis macularia)•••507 Upland Sandpiper (Bartramia longicauda)•509 Western Sandpiper (Calidris mauri) •••481 Wilson’s Phalarope (Phalaropus tricolor) ••515 All illustrations in these profiles are copyrighted © George C. West, and used with permission. To view his work go to http://www.birchwoodstudio.com. S H O R E B I R D S M 472 I Explore the World with Shorebirds! S A T R ER G S RO CHOOLS P Red Knot (Calidris canutus) Description The Red Knot is a chunky, medium sized shorebird that measures about 10 inches from bill to tail. When in its breeding plumage, the edges of its head and the underside of its neck and belly are orangish. The bird’s upper body is streaked a dark brown. It has a brownish gray tail and yellow green legs and feet. In the winter, the Red Knot carries a plain, grayish plumage that has very few distinctive features. Call Its call is a low, two-note whistle that sometimes includes a churring “knot” sound that is what inspired its name. -

Iucn Red Data List Information on Species Listed On, and Covered by Cms Appendices
UNEP/CMS/ScC-SC4/Doc.8/Rev.1/Annex 1 ANNEX 1 IUCN RED DATA LIST INFORMATION ON SPECIES LISTED ON, AND COVERED BY CMS APPENDICES Content General Information ................................................................................................................................................................................................................................ 2 Species in Appendix I ............................................................................................................................................................................................................................... 3 Mammalia ............................................................................................................................................................................................................................................ 4 Aves ...................................................................................................................................................................................................................................................... 7 Reptilia ............................................................................................................................................................................................................................................... 12 Pisces ................................................................................................................................................................................................................................................. -

Laughing Gulls Breed Primarily Along the Pacific Coast of Mexico and the Atlantic and Caribbean Coasts from S
LAUGHING GULL Leucophaeus atricilla non-breeding visitor, regular winterer L.a. megalopterus Laughing Gulls breed primarily along the Pacific coast of Mexico and the Atlantic and Caribbean coasts from s. Canada to Venezuela, and they winter S to Peru and the Amazon delta (AOU 1998, Howell and Dunn 2007). It and Franklin's Gull were placed along with other gulls in the genus Larus until split by the AOU (2008). Vagrant Laughing Gulls have been reported in Europe (Cramp and Simmons 1983) and widely in the Pacific, from Clipperton I to Wake Atoll (Rauzon et al. 2008), Johnston Atoll (records of at least 14 individuals, 1964-2003), Palmyra, Baker, Kiribati, Pheonix, Marshall, Pitcairn, Gambier, and Samoan Is, as well as Australia/New Zealand (King 1967; Clapp and Sibley 1967; Sibley and McFarlane 1968; Pratt et al. 1987, 2010; Garrett 1987; Wragg 1994; Higgins and Davies 1996; Vanderwerf et al. 2004; Hayes et al. 2015; E 50:13 [identified as Franklin's Gull], 58:50). Another interesting record is of one photographed attending an observer rowing solo between San Francisco and Australia at 6.5° N, 155° W, about 1400 km S of Hawai'i I, 1-2 Nov 2007. They have been recorded almost annually as winter visitors to the Hawaiian Islands since the mid-1970s, numbers increasing from the NW to the SE, as would be expected of this N American species. The great majority of records involve first-year birds, and, despite the many records in the S Pacific, there is no evidence for a transient population through the Hawaiian Islands, or of individuals returning for consecutive winters after departing in spring. -

First Confirmed Record of Belcher's Gull Larus Belcheri for Colombia with Notes on the Status of Other Gull Species
First confirmed record of Belcher's Gull Larus belcheri for Colombia with notes on the status of other gull species Primer registro confirmado de la Gaviota Peruana Larus belcheri para Colombia con notas sobre el estado de otras especies de gaviotas Trevor Ellery1 & José Ferney Salgado2 1 Independent. Email: [email protected] 2 Corporación para el Fomento del Aviturismo en Colombia. Abstract We present photographic records of a Belcher's Gull Larus belcheri from the Colombian Caribbean region. These are the first confirmed records of this species in the country. Keywords: new record, range extension, gull, identification. Resumen Presentamos registros fotograficos de un individuo de la Gaviota Peruana Larus belcheri en la region del Caribe de Colombia. Estos son los primeros registros confirmados para el país. Palabras clave: Nuevo registro, extensión de distribución, gaviota, identificación. Introduction the Pacific Ocean coasts of southern South America, and Belcher's Gull or Band-tailed Gull Larus belcheri has long Olrog's Gull L. atlanticus of southern Brazil, Uruguay and been considered a possible or probable species for Argentina (Howell & Dunn 2007, Remsen et al. 2018). Colombia, with observations nearby from Panama (Hilty & Brown 1986). It was first listed for Colombia by Salaman A good rule of thumb for gulls in Colombia is that if it's not et al. (2001) without any justification or notes, perhaps on a Laughing Gull Leucophaeus atricilla, then it's interesting. the presumption that the species could never logically have A second good rule of thumb for Colombian gulls is that if reached the Panamanian observation locality from its it's not a Laughing Gull, you are probably watching it at Los southern breeding grounds without passing through the Camarones or Santuario de Fauna y Flora Los Flamencos, country. -
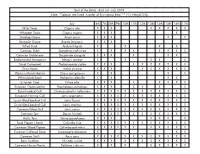
Best of the Baltic - Bird List - July 2019 Note: *Species Are Listed in Order of First Seeing Them ** H = Heard Only
Best of the Baltic - Bird List - July 2019 Note: *Species are listed in order of first seeing them ** H = Heard Only July 6th 7th 8th 9th 10th 11th 12th 13th 14th 15th 16th 17th Mute Swan Cygnus olor X X X X X X X X Whopper Swan Cygnus cygnus X X X X Greylag Goose Anser anser X X X X X Barnacle Goose Branta leucopsis X X X Tufted Duck Aythya fuligula X X X X Common Eider Somateria mollissima X X X X X X X X Common Goldeneye Bucephala clangula X X X X X X Red-breasted Merganser Mergus serrator X X X X X Great Cormorant Phalacrocorax carbo X X X X X X X X X X Grey Heron Ardea cinerea X X X X X X X X X Western Marsh Harrier Circus aeruginosus X X X X White-tailed Eagle Haliaeetus albicilla X X X X Eurasian Coot Fulica atra X X X X X X X X Eurasian Oystercatcher Haematopus ostralegus X X X X X X X Black-headed Gull Chroicocephalus ridibundus X X X X X X X X X X X X European Herring Gull Larus argentatus X X X X X X X X X X X X Lesser Black-backed Gull Larus fuscus X X X X X X X X X X X X Great Black-backed Gull Larus marinus X X X X X X X X X X X X Common/Mew Gull Larus canus X X X X X X X X X X X X Common Tern Sterna hirundo X X X X X X X X X X X X Arctic Tern Sterna paradisaea X X X X X X X Feral Pigeon ( Rock) Columba livia X X X X X X X X X X X X Common Wood Pigeon Columba palumbus X X X X X X X X X X X Eurasian Collared Dove Streptopelia decaocto X X X Common Swift Apus apus X X X X X X X X X X X X Barn Swallow Hirundo rustica X X X X X X X X X X X Common House Martin Delichon urbicum X X X X X X X X White Wagtail Motacilla alba X X -

Florida Beach-Nesting Bird Report, 2005-2008
Florida Beach-nesting Bird Report Summary of FWC’s Beach-nesting Bird Database from 2005-2008 Prepared by Chris Burney FWC Shorebird Partnership Coordinator [email protected] FLORIDA BNB REPORT 2005-2008 2 3 1 Florida Beach-nesting Bird Report A look back at the data collected from 2005 to 2008 and a look forward at considerations for the future. INTRODUCTION we cannot interpret these data in the same way we would those For those of you regularly submitting data online to the Florida from a systematic survey. This means that you should use a great Beach-nesting Bird (BNB) database, you are probably very fa- deal of caution in interpreting the tables and maps presented in miliar with the activity on your stretch of beach but not sure how this report. For instance, we definitely can not draw conclusions the birds and sites you monitor factor into the bigger picture. In about population status and trends (e.g. how many Least Terns view of this, we have produced the following report which sum- nest in Florida, and whether or not they are declining) from such marizes the data compiled in the BNB database from 2005 to assorted data. Despite these limitations, the BNB database does 2008. We hope that it will provide you, our contributors, a more function as a resource for current information about the distri- statewide perspective. These data would certainly not be avail- bution (i.e. where birds are nesting) and relative abundance (i.e. able if not for the dedicated work of our partners from federal, how many pairs are nesting compared to other sites) of beach- state, and local governments to private conservation organiza- nesters and provides valuable information for developing re- tions and individuals.