[Houston Ship Channel and Galveston Bay Dioxin Study]
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-
Facility Location Codes Advent, Inc
Facility Location Codes Advent, Inc. 908.795.3223 DRS LOC CODE Facility Name Address City State Zip USLAX3PL 3PLUS LOGISTICS CO 20250 S ALAMEDA ST. RANCHO DOMINGUEZ CA USBALAHG A H GARDNER & SON 2207 S. NEWKIRK ST. BALTIMORE MD 21224 USLAXAMC A M CONTAINERS SERVICE 505 EAST G WILMINGTON CA USPHXAIP ABSOLUTE INTERMODAL 6602 W. GRANT ST. PHOENIX AZ 85043 USCHIACE ACE WORLD WIDE 1901 E. ACE WORLDWIDE LANE CUDAHY WI USELPACI ADVANCE CONTAINER INC. EL PASO 7180 COOPERQUEEN DR. EL PASO TX USDFWACI ADVANCE CONTAINER, INC. FORT WORTH 920 NORTH MAIN FORT WORTH TX 76035 USHOUACH ADVANCED CONTAINER, INC. HOUSTON 1715 TURNING BASIN DR. HOUSTON TX 77029 USSEAAFF AFFORDABLE STORAGE 1721 THORNE RD. TACOMA WA 98421 USSEAASC AFFORDABLE STORAGE CONTAINER 2308 MILWAUKEE WAY TACOMA WA 98421 USSTLATS AFFTON TERMINAL SERVICES 420 GIMBLIN RD. ST. LOUIS MO 63147 USPHLAIY AGX INTERMODAL YARD PITTSBURGH 901 MOSSIDE BLVD WALL PA USCHIAGX AIM TRANSFER & STORAGE 7774 S 10TH ST. OAK CREEK WI 53154 USOAKASO AIR SEAS 370 8TH AVE. OAKLAND CA 94606 USANCAGR ALASKA GLACIER REFRESHMENTS INC. 21800 EKLUTNA LAKE RD. CHUGIAK AK USANCAMH ALASKA MARINE LINE - BARGE SERVICE T.J.K. FISHERIES HAINES AK USANCAMK ALASKA MARINE LINE (YAKATAT) C/O AML (KETCHIKAN) KETCHIKAN AK USANCAMJ ALASKA MARINE LINE JUNEAU- C/O JAMESTOWN BAY WHSE 550 S. FRANKLIN JUNEAU AK 99801 USANCAMS ALASKA MARINE LINE SITKA- C/O JAMESTOWN BAY WHSE 1517 SAWMILL CREEK RD. SITKA AK 99835 USANCAMW ALASKA MARINE LINE WRANGELL OFFICE 520 FRONT ST. WRANGELL AK 99929 USANCAMC ALASKA MARINE LINES, INC. 202 INDUSTRY RD. CORDOVA AK 99574 USANCAMT ALASKA MARINE LINES-CRAIG C/O ARROWHEAD TRANSFER C/O ARROWHEAD TRANSFER CRAIG AK 99921 USANCAM2 ALASKA MARINE LINES-KETCHICAN- C/O JAMESTOWN BAY WHSE 3295 TONGASS AVE. -

Baytown Mobility Plan ACKNOWLEDGEMENTS
BAYTOWN MOBILITY PLAN u Adopted January 24, 2013 City of Baytown Baytown Mobility Plan ACKNOWLEDGEMENTS City Council – Mayor, Stephen H. DonCarlos 2011 Members District 1 – Mercedes Renteria III Barry Bobbitt District 2 – Scott Sheley Brandon Benoit District 3 – Brandon Capetillo Gil Chambers District 4 – Terry Sain Dr. Joe C. Floyd District 5 – Robert C. Hoskins Jim Hutchison District 6 – David McCartney Mary Flores Planning & Zoning Commission Rolando Valdez Tracey Wheeler 2012 Members Spencer Carnes Brandon Benoit City Staff R.D. Burnside Administration Spencer Carnes Bob Leiper, City Manager Gilbert Chambers Ron Bottoms, Deputy City Manager James E. “Bo” Cox Kevin Troller, Assistant City Manager Lisa D. Clary Dr. Joe C. Floyd Planning & Development Services Kelly Carpenter, AICP, Director of Planning and Development Shawn McDonald Services Tracey Wheeler Tiffany Foster, AICP, Assistant Director Andrew Allemand, AICP, Development Review Manager Page – i City of Baytown Baytown Mobility Plan TABLE OF CONTENTS ACKNOWLEDGEMENTS ............................................................................ i 6.5 Conclusions ............................................................................... 6-10 LIST OF ACRONYMS ............................................................................... iv CHAPTER 7. MULTI-MODAL ASSESSMENT ............................................. 7-1 CHAPTER 1. INTRODUCTION ................................................................ 1-1 7.1 Introduction ............................................................................... -

DIRECT ECONOMIC EFFECTS of LACK of MAINTENANCE DREDGING of the HOUSTON SHIP CHANNEL December 2010
DIRECT ECONOMIC EFFECTS OF LACK OF MAINTENANCE DREDGING OF THE HOUSTON SHIP CHANNEL December 2010 Prepared by CENTER FOR PORTS AND WATERWAYS TEXAS TRANSPORTATION INSTITUTE 701 NORTH POST OAK, SUITE 430 HOUSTON, TEXAS 77024‐3827 for PORT OF HOUSTON AUTHORITY Table of Contents EXECUTIVE SUMMARY ............................................................................................................. i CHAPTER 1: BACKGROUND AND PROJECT APPROACH .................................................. 1 Phase 1 ........................................................................................................................................ 3 Phase 2 ........................................................................................................................................ 6 CATEGORY 1: LIGHT LOADING ........................................................................................... 10 Selection and Data Acquisition ................................................................................................. 10 Valuation ................................................................................................................................... 13 CATEGORY 2: PARTIAL DISCHARGE AT WOODHOUSE TERMINAL ........................... 17 Selection and Data Acquisition ................................................................................................. 17 Valuation ................................................................................................................................... 17 CATEGORY -

Port of Houston Authority Tariff No. 14
Port of Houston Authority Tariff No. 14 February 1, 2021 Additional Rates, Rules, and Regulations Governing the Fentress Bracewell Barbours Cut Container Terminal EXECUTIVE OFFICES: 111 East Loop North - Houston, Texas 77029 USA P. O. Box 2562 - Houston, Texas 77252-2562 Phone (713) 670-2400 - Fax (713) 670-2564 Barbours Cut Container Terminal 1515 East Barbours Cut Boulevard – La Porte, Texas 77571 Phone (281) 470-1800 - Fax (281) 470-5580 PORT OF HOUSTON TARIFF NO. 14 Page No. 2 TABLE OF CONTENTS SECTION ONE: DEFINITIONS AND ABBREVIATIONS SUBJECT SUBRULE PAGE NO. Abbreviations ........................................................................................................... 048 ......................................... 11 Agent or Vessel Agent ............................................................................................. 001 .......................................... 6 Baplie ....................................................................................................................... 002 ........................................... 6 Berth ......................................................................................................................... 003 ........................................... 6 Bonded Storage ....................................................................................................... 004 ........................................... 6 Checking .................................................................................................................. 005 -

Port Houston 2040 Plan Comprehensive Long-Range Plan
PORT HOUSTON 2040 PLAN COMPREHENSIVE LONG-RANGE PLAN READY FOR THE FUTURE CONTENTS WE ARE READY FOUNDATION FOR THE FUTURE BUSINESS RESILIENCE DISCLAIMER INTRODUCTION TO THE 2040 PLAN THE PLANS OF PORT HOUSTON IMPLEMENTING THE 2040 PLAN This document has been prepared for Port 01 03 05 Houston’s planning purposes only. 1 LETTER FROM THE EXECUTIVE DIRECTOR 19 FOUNDATION FOR THE FUTURE 53 ANTICIPATING AND PREPARING FOR THE FUTURE No person or organization should rely upon 2 EXECUTIVE SUMMARY 20 A COLLECTIVE COMMITMENT the information contained in this document. 54 INFORMED, FLEXIBLE, ADAPTABLE IMPLEMENTATION Moreover, no person or organization should 3 ABOUT THE 2040 PLAN 21 STRATEGIC PLAN 55 CAPITAL INVESTMENT rely upon expectations of Port Houston to 22 KEEPING PEOPLE FIRST meet the strategic objectives or otherwise 23 PLANS TRANSLATE VISION INTO ACTION take any action outlined in this document. Any potential infrastructure development 24 THE FOUR Cs OF THE 2040 PLAN identified in this document will be subject to financial viability, technical feasibility, and environmental and planning considerations, which may vary over time. No commercial PAST AND PRESENT THE FOUR Cs DRIVING REGIONAL PROSPERITY reliance may be placed on any forecasts or 02 AN IRREPLACEABLE PORT 04 2040 PLAN STRATEGIES 06 PARTNERSHIPS FOR COMMON GOALS projects referred to in this document. 7 A PORT AS BIG AS TEXAS 27 CHANNEL 59 DELIVERING RESULTS DELIVERS VALUE ACKNOWLEDGMENTS 9 THE JOURNEY CONTINUES 33 CARGO 60 COOPERATION COORDINATION COLLABORATION Port Houston would like to recognize 11 THE INTERNATIONAL PORT OF TEXAS 39 COMMUNITY 61 2040 PLAN IN CONTEXT the contributions made by its staff, Port Commission, customers, stakeholders, and its 12 AN ESTABLISHED GATEWAY 45 CHANGE 63 A GROWING REGION consultants SWA Group and CAS Group in the 13 PORT HOUSTON ROLES development of this document. -

A Public Meeting of the Port Commission of the Port of Houston
Port of Houston Authority Houston, Texas Port Commission Public Meeting January 28, 2020 A public meeting of the Port Commission of the Port of Houston Authority of Harris County, Texas was convened on January 28, 2020 at 9:01 a.m., at the Port of Houston Authority Executive Office, Fourth Floor Boardroom, at 111 East Loop North, Houston, Texas 77029. The following commissioners, staff, and counsel were present: Ric Campo, Chairman Theldon R. Branch, III, Commissioner Wendy Cloonan, Commissioner Dean E. Corgey, Commissioner Stephen H. DonCarlos, Commissioner Clyde E. Fitzgerald, Commissioner Roy D. Mease, Commissioner Roger Guenther, Executive Director Erik Eriksson, Chief Legal Officer Tom Heidt, Chief Operating Officer J. Kent Friedman, outside counsel Chairman Campo convened the meeting by welcoming everyone to the twelfth decade of the Port Commission. He noted that the Strategic Plan was on the agenda for approval and highlighted its four strategic goals, placing an emphasis on the first goal: People. Chairman Campo recognized that staff makes the Port Authority what it is, added that one of the goals was to make the Port Authority one of the best places to work, and to continue to do that, a great culture needed to be cultivated, and pay and benefits must also be competitive. Chairman Campo recognized Infrastructure as the next goal, noting that this goal was directly tied to deepening and widening the Houston Ship Channel and emphasizing that the project needed the Chief’s Report and Water Resources Development Act (WRDA) approval in 2020. He added that financing must also get worked out to allow the project to be completed on a fast track. -

Applicability of the Technologies of Intelligent Transportation Systems
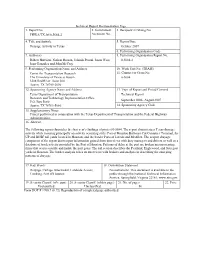
Technical Report Doeumentatlon Paee 1. Report No. .f 2. Govenunent Accession No. 3. Recipient's Catalog No. SWUTC/951721912-2 4. Title and Subtitle S. Report Date Applicability of the Technologies ofIntelligent Transportation Systems February 1995 in Commercial Vehicle Operations at the Port of Houston's Intermodal 6. Perfonning Organization Code Marine Container Terminal with Case Studies of Existing Systems 7. Author(s) 8. Performing Organization Report No. Richard B. Easley and C. Michael Walton 9. Perfonning Organization Name and Address 10. Work Unit No. (TRAlS) Center for Transportation Research The University of Texas at Austin 11. Contract or Grant No. 3208 Red River, Suite 200 DTOS88-G-0006 Austin, Texas 78705-2650 12. Sponsoring Agency Name and Address 13. Type of Report and Period Covered Southwest Region University Transportation Center Texas Transportation Institute The Texas A&M University System 14. Sponsoring Agency Code College Station, Texas 77843-3135 15. Supplementary Notes Supported by a grant from the U.S. Department of Transportation, University Transportation Centers Program. 16. Abstract The scope of the research includes identifying problems with the Barbours Cut Container Tenninal gate operations problems and developing a viable solution to remedy those problems. The study will consist of a detailed analysis of Barbours Cut's gate operations and will introduce various alternatives with the objective of reducing truck in-tenninal dwell times (sometimes called 'truck tum times'). Included among the alternatives -

Rates Reduced at Barbours Cut Terminal A
Tariff change Rates reducedat BarboursCut Terminal 10 percent reduction in the tariff on loaded con- they are provisional in nature." tainer throughput charges at Barbours Cut Terminal The new throughput charge is $54 per loaded con- Ahas been announced by the Port of Houston tainer, for both imports and exports. Authority. The rental rate for a yard tractor and chassis as one In addition, for containers on which throughput unit has been changed to $20 per unit per hour, while charges are paid, no fee will be charged for drayage be- rental for a heavy-duty RO/ROtractor is now $30 per tween the container park or roll-on/roll-off staging area unit per hour. Rental for a yard tractor alone is $15 per and the Barbours Cut rail ramp, which is located con- unit per hour, and rental for a chassis costs $5 per unit veniently inside the terminal. per hour. Finally, rental rates have been reduced for various Barbours Cut Terminal handles both containerized pieces of equipment. and roll-on/roll-off cargo, and has eight wharf cranes, 11 "These changes are being made to reduce the costs in- yard cranes, a RO/ROramp and 100,000 square feet of curred by steamship lines calling at Barbours Cut," said covered storage. Each of its four berths is backed with R.P. Leach, executive director of the Port of Houston more than 36 acres of paved marshalling area for con- Authority. "The elimination of the drayage charge tainers and RO/ROcargo. should prove particularly attractive to shippers who "Barbours Cut is an efficient intermodal gateway to want to use a landbridge to and from the U.S. -

For Sale Or Lease Baytown, TX 77521
Lake Conroe Willis Cleveland 105 105 Conroe Not actual building The Woodlands Thompson Ten - Ratcliff Reserve I-10 East at Thompson Rd For SaleTomball or Lease BaytoKingwoodwn, TX 77521 +15,000 s.f. crane ready building on ±1.0 acres Richard Quarles, SIOR +1 713 888 4019 Site features: Bush [email protected] Intercontinental – ± 15,000 s.f. crane-ready building on ±1.0 Airport Joe Berwick acres +1 713 425 5842 – O ice: 10% inish [email protected] – Crane capacity: 10 ton – Eave height: 28’ Site – Power: 400 amps, 480V, 3-phase Katy Thompson Rd – Overhead doors: (2) 14’ x 16’ Baytown – Utilities in place Houston – Regional detention provided Barbours Cut Terminal – Truck court: ±100 – Under construction - completion Q1 2019 La Porte Pasadena – Up to ±6.75 acres available for expansion Hobby Airport or build-to-suit 146 Sugar Land Ellington Galveston Owned and developed by: Field Bay Missouri City Bayport Container Terminal Pearland League City +15,000 s.f. crane ready building on ±1.0 acres ForSale or Lease Baytown, TX 77521 Location Located just south of I-10 at the Thompson Road exit, The Ratcliff Reserve at Thompson 10 is conveniently located near major refineries and petrochemical plants locations as well as Port of Houston terminals. Via Major Highways East Freeway Frontage Road Gilbert Landing LOCATION APPROX. DRIVE TIME San Jacinto Mall Site Exxon 6 minutes WADE RD WADE Barbours Cut Terminal 10 minutes Chevron 14 minutes Grand Parkway 14 minutes THOMPSON RD Bayport Terminal 15 minutes Jacintoport Terminal 15 minutes UPRR COADY RAIL YARD Greensport Terminal 18 minutes Shell 18 minutes Lynchburg Lyondell 19 minutes Total 19 minutes 225330 Bush Intercontinental Airport 32 minutes Hobby Airport 27 minutes CHEVRON TO BUSH Site INTERCONTINENTAL AIRPORT 1000 yd Oct 12 2017 © Stepmap, 123map • Data: OpenStreetMap, License ODbL 1.0 BAYTOWN 225330 JACINTOPORT EXXON HOUSTON TERMINAL GREENSPORT TERMINAL 1468 SHELL 225 225146 BARBOURS CUT TERMINAL HOBBY AIRPORT LYONDELL +15,000 s.f. -
Drayage Activity in Texas October 2007 6
Technical Report Documentation Page 1. Report No. 2. Government 3. Recipient’s Catalog No. FHWA/TX-08/0-5684-2 Accession No. 4. Title and Subtitle 5. Report Date Drayage Activity in Texas October 2007 6. Performing Organization Code 7. Author(s) 8. Performing Organization Report No. Robert Harrison, Nathan Hutson, Jolanda Prozzi, Jason West, 0-5684-2 Juan Gonzalez and John McCray 9. Performing Organization Name and Address 10. Work Unit No. (TRAIS) Center for Transportation Research 11. Contract or Grant No. The University of Texas at Austin 0-5684 3208 Red River, Suite 200 Austin, TX 78705-2650 12. Sponsoring Agency Name and Address 13. Type of Report and Period Covered Texas Department of Transportation Technical Report Research and Technology Implementation Office September 2006- August 2007 P.O. Box 5080 Austin, TX 78763-5080 14. Sponsoring Agency Code 15. Supplementary Notes Project performed in cooperation with the Texas Department of Transportation and the Federal Highway Administration. 16. Abstract The following report chronicles the first year’s findings of project 0-5684. The report characterizes Texas drayage activity while focusing principally on activity occurring at the Port of Houston Barbours Cut Container Terminal, the UP and BNSF rail yards located in Houston, and the border Ports of Laredo and McAllen. The seaport drayage component of the report draws upon information gained from interviews with dray managers and drivers as well as a database of truck activity provided by the Port of Houston. Patterns of delay at the port are broken into processing times that accrue outside and inside the port gates. -

July 28, 2021 – Port Houston Reopens Container Terminals
For immediate release July 28, 2021 PORT HOUSTON REOPENS CONTAINER TERMINALS Hardware Matter is Resolved BAYPORT TERMINAL PERMIT IS RENEWED AS IMPORTS CONTINUE TO POUR IN AND JUNETEENTH HOLIDAY RECOGNIZED Photo Caption: Port Houston’s busy Bayport Container Terminal HOUSTON –Truck gate operations have resumed Thursday afternoon at Barbours Cut and Bayport Terminals, following resolution of computer hardware issues that closed both terminals to truck traffic for two and a half days. “Extended gate times are planned through the weekend - or as long as needed – until business is caught up,” Port Houston Executive Director Roger Guenther said. Guenther expressed regret to customers and partners, including truckers, and appreciation for their patience while resolving the issue. Guenther said, “We will take a strong look to find what caused the problem, and measures will be put in place to help prevent it from happening again.” In more Port Houston news, the U.S. Army Corps of Engineers renewed the federal permit for Port Houston’s Bayport Terminal. This significant milestone was officially announced and applauded during the July meeting of the Port Commission of the Port of Houston Authority held last week. This crucial permit allows Port Houston to continue its master plan for construction to build out the Bayport Terminal, which includes its current $200 million expansion program to complete Wharf 6, Container Yard 2, and other projects – part of Port Houston continuing efforts to remain ahead of accelerating demand. Port Chairman Ric Campo also announced Port Houston was on track in the coming weeks to enter into a Project Partnership Agreement or PPA with the Army Corps, a key step in Project 11 widening construction program of the Houston Ship Channel. -

Barbours Cut Safety Manual
Port Houston Health, Safety, Security, and Environment Policy Document Document Control # HSSE-SAF-402 Revision Date 04/26/2017 Document Title Barbours Cut Truck Driver Safety Manual Authored by C. Armenoff Document Level Level 4-Truck Driver Safety Manual Reviewed by J. Abril Document Owner HSSE Approved by J. Davis 1. Purpose 1.1. Port Houston is committed to providing a safe and healthy place of business. In an effort to meet this goal, the PORT HOUSTON has developed this Barbours Cut Terminal Trucking Industry Safety Manual (“Safety Manual”) to communicate safety procedures, standards and work related rules to all drivers operating at the Barbours Cut. The cooperation of each driver in following the rules contained in the Safety Manual is required for the PORT HOUSTON to achieve its safety goals. The procedures, standards and rules contained in the Safety Manual apply to all truck drivers operating at Barbours Cut Terminal. 2. Scope 2.1. The Safety Manual applies to all areas of Bay Port Container Terminal. 2.2. The Safety Manual will not cover every possible situation that drivers may encounter while at Barbours Cut Terminal. 2.3. Drivers must exercise good judgment at all times and be responsible for their own safety. 2.4. All drivers are responsible for knowing and following all safety procedures, standards, rules and policies. 2.5. Disorderly or unsafe conduct, failure to follow safe work practices, or violation of or refusal to comply with PORT HOUSTON safety rules can be cause for removal of driving privileges and may result in the driver being denied access to Barbours Cut Container Terminals.