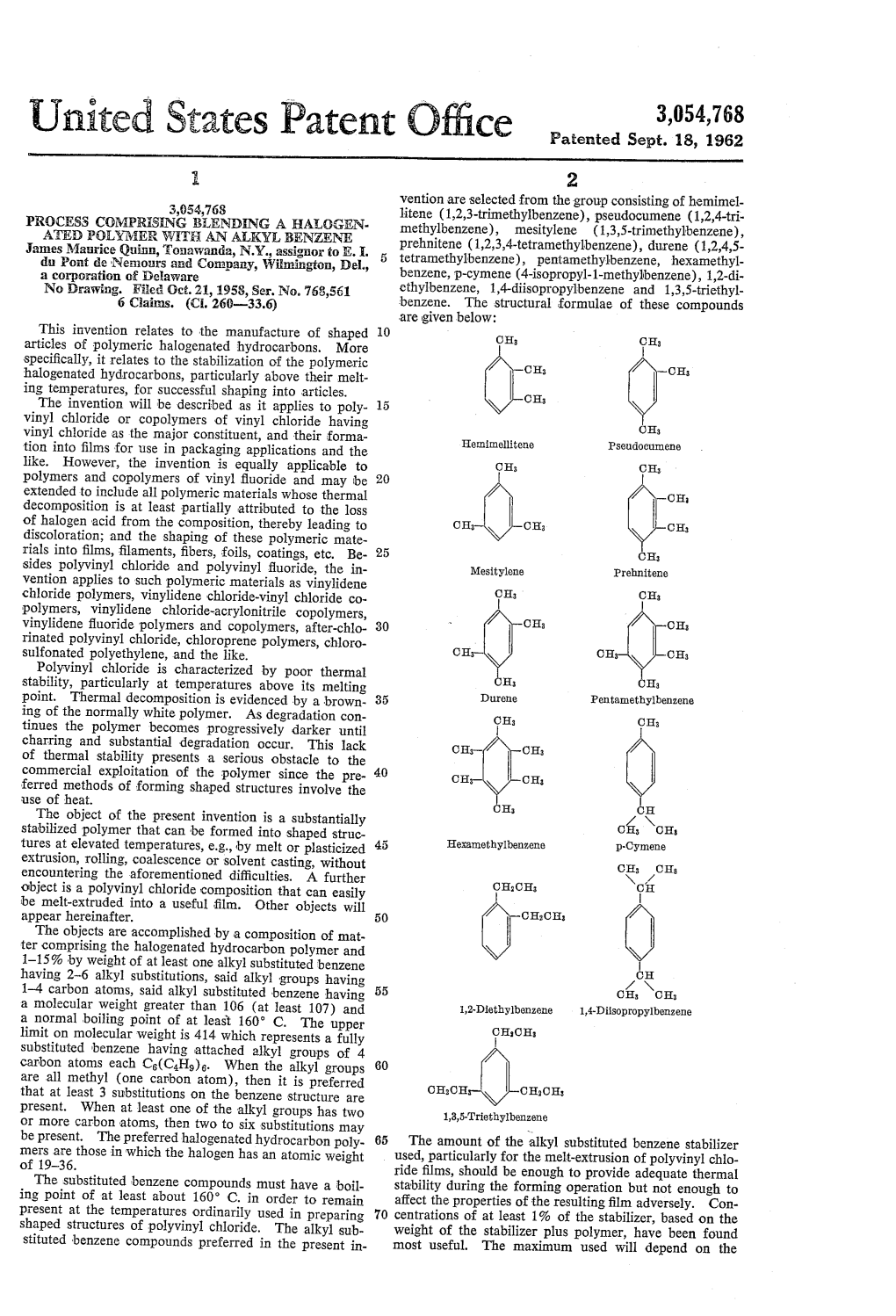
United States Patent Office Patented Sept
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Aldrich Vapor
Aldrich Vapor Library Listing – 6,611 spectra This library is an ideal tool for investigator using FT-IR to analyze gas phase materials. It contains gas phase spectra collected by Aldrich using a GC-IR interface to ensure chromatographically pure samples. The Aldrich FT-IR Vapor Phase Library contains 6,611 gas phase FT-IR spectra collected by Aldrich Chemical Company using a GC interface. The library includes compound name, molecular formula, CAS (Chemical Abstract Service) registry number, Aldrich catalog number, and page number in the Aldrich Library of FT-IR Spectra, Edition 1, Volume 3, Vapor-Phase. Aldrich Vapor Index Compound Name Index Compound Name 6417 ((1- 3495 (1,2-Dibromoethyl)benzene; Styrene Ethoxycyclopropyl)oxy)trimethylsilane dibromide 2081 (+)-3-(Heptafluorobutyryl)camphor 3494 (1-Bromoethyl)benzene; 1-Phenylethyl 2080 (+)-3-(Trifluoroacetyl)camphor bromide 262 (+)-Camphene; 2,2-Dimethyl-3- 6410 (1-Hydroxyallyl)trimethylsilane methylenebicyclo[2.2.1]heptane 6605 (1-Methyl-2,4-cyclopentadien-1- 2828 (+)-Diisopropyl L-tartrate yl)manganese tricarbonyl 947 (+)-Isomenthol; [1S-(1a,2b,5b)]-2- 6250 (1-Propynyl)benzene; 1-Phenylpropyne Isopropyl-5-methylcyclohexano 2079 (1R)-(+)-3-Bromocamphor, endo- 1230 (+)-Limonene oxide, cis + trans; (+)-1,2- 2077 (1R)-(+)-Camphor; (1R)-(+)-1,7,7- Epoxy-4-isopropenyl-1- Trimethylbicyclo[2.2.1]heptan- 317 (+)-Longifolene; (1S)-8-Methylene- 976 (1R)-(+)-Fenchyl alcohol, endo- 3,3,7-trimethyltricyclo[5.4.0 2074 (1R)-(+)-Nopinone; (1R)-(+)-6,6- 949 (+)-Menthol; [1S-(1a,2b,5a)]-(+)-2- Dimethylbicyclo[3.1.1]heptan-2- -

Llllllillllllllllillllllllllllllllllllllllllllilllllllllllll
llllllIllllllllllIllllllllllllllllllllllllllllIlllllllllllllllllllllllllllSOO5223589A United States Patent -- [19] [11] Patent Number: 5,223,589 Kulprathipanja et al. [45] Date of Patent: Jun. 29, 1993 [54] PROCESS FOR SEPARATING DURENE 3.422.848 1/1969 Liebman et a]. ......... .. 137/625.l5 FROM SUBSTITUTED BENZENE 3.706.812 12/1972 De Rosset et a1. 260/674 SA v 3,864,416 2/1975 Campbell et al. .. 260/674 A HYDROCARBOI\S 4.642.397 2/1987 Zinnen et al. .. 568/934 [75] Inventors: Santi Kulprathipanja, Inverness; 4,743.708 5/1988 Rosenfeld et al. ................ .. 585/828 Kenneth K. Kuhnle, St. Charles; Marjorie s. Patton, Darien, all of 111. OTHER PUBLICATIONS , . Chartov et al. Chem. Abstract 92(7) 58328d [73] Asslgnee: UOP’ Des Flames’ 111‘ (l972)-—Abstract only, page unavailable. [21] APPL No-i 872,191 Primary Examiner-Helen M. S. Sneed [22] Filed. Apr. 22, 1992 Assistant Examiner-Nhat D. Phan Attorney, Agent, or Firm-Thomas K. McBride; John F. [51] Int. (31.5 ......................... .. C07C 7/12; C07C 7/00 Spears’ 1L; Jack H_ Hall - [52] US. Cl. .................................. .. 585/828; 585/825; 585/831; 585/853 [57] ABSTRACT [58] Field Of Search .... ........ .. 585/825. 828, 831, 853 The separation Ofdurene in purity from coal tar or . petroleum fractions by an adsorptive chromatographic [56] References cued process and liquid phase with lithium-exchanged X US- PATENT DOCUMENTS zeolite as the adsorbent and liquid aromatic desorbents. 2.985.589 5/1961 Broughton er al. ................ .. 210/34 3.040.777 6/1962 Carson et al. ................ .. l37/625.l5 4 Claims, No Drawings 5,223,589 1 2 scale (deRosset U.S. -

Title Unusual Aromatic Nitrations
Unusual Aromatic Nitrations (Commemoration Issue Title Dedicated to Professor Sango Kunichika On the Occasion of his Retirement) Author(s) Suzuki, Hitomi Bulletin of the Institute for Chemical Research, Kyoto Citation University (1972), 50(4): 407-422 Issue Date 1972-11-17 URL http://hdl.handle.net/2433/76436 Right Type Departmental Bulletin Paper Textversion publisher Kyoto University Bull. Inst. Chem. Res., Kyoto Univ., Vol. 50, No. 4, 1972 Unusual Aromatic Nitrations Hitomi S uzu Kt* ReceivedApril 30, 1972 Aromaticcompounds undergo three differenttypes of reactions with nitrating agentsunder ionic conditions;replacement by nitro groupof an atom or groupfrom a ring position(ordinary nitration), reaction on substituentgroups, and addition reactionfollowed by various transformations. The presentsurvey is directedtowards the latter two typesof reactions,which have hithertonot yet been summarizedin chemicalliterature. It includes; 1. Reactionson SubstituentGroups. 1.1 Side-chainNitro-oxylation. 1.2 Side-chainAcetoxylation and Acetamidation. 1.3 Side-chainNitration. 1.4 Reactionson Lateral Nitrogenor OxygenAtoms. 2. Reactionson AromaticRing. 2.1 Acyloxylationand Alkoxylation. 2.2 Oxynitration. 2.3 Formation of UnsaturatedCyclic Ketones. 2.4 NitrativeCondensations. 2.5 Nitrations with Rearrangement. Nitration is one of the most basic reactions in organic chemistry and is widely used for the preparation of nitro compounds which are among the most valuable intermediates in organic synthesis. The compounds to be nitrated may be either aliphatic or aromatic, but the reaction has more significance in aromatic chemistry. Aromatic nitration is the process in which the nitro group replaces an atom or group from a ring position of an aromatic compound. The reaction has already been dealt with by a number of reviews and books)) In recent years, however, several new reactions have come to light which give the results considerably deviated from the ordinary concept of aromatic nitration. -

"Hydrocarbons," In: Ullmann's Encyclopedia of Industrial Chemistry
Article No : a13_227 Hydrocarbons KARL GRIESBAUM, Universit€at Karlsruhe (TH), Karlsruhe, Federal Republic of Germany ARNO BEHR, Henkel KGaA, Dusseldorf,€ Federal Republic of Germany DIETER BIEDENKAPP, BASF Aktiengesellschaft, Ludwigshafen, Federal Republic of Germany HEINZ-WERNER VOGES, Huls€ Aktiengesellschaft, Marl, Federal Republic of Germany DOROTHEA GARBE, Haarmann & Reimer GmbH, Holzminden, Federal Republic of Germany CHRISTIAN PAETZ, Bayer AG, Leverkusen, Federal Republic of Germany GERD COLLIN, Ruttgerswerke€ AG, Duisburg, Federal Republic of Germany DIETER MAYER, Hoechst Aktiengesellschaft, Frankfurt, Federal Republic of Germany HARTMUT Ho€KE, Ruttgerswerke€ AG, Castrop-Rauxel, Federal Republic of Germany 1. Saturated Hydrocarbons ............ 134 3.7. Cumene ......................... 163 1.1. Physical Properties ................ 134 3.8. Diisopropylbenzenes ............... 164 1.2. Chemical Properties ............... 134 3.9. Cymenes; C4- and C5-Alkylaromatic 1.3. Production ....................... 134 Compounds ...................... 165 1.3.1. From Natural Gas and Petroleum . .... 135 3.10. Monoalkylbenzenes with Alkyl Groups 1.3.2. From Coal and Coal-Derived Products . 138 >C10 ........................... 166 1.3.3. By Synthesis and by Conversion of other 3.11. Diphenylmethane .................. 167 Hydrocarbons . .................. 139 4. Biphenyls and Polyphenyls .......... 168 1.4. Uses ............................ 140 4.1. Biphenyl......................... 168 1.5. Individual Saturated Hydrocarbons ... 142 4.2. Terphenyls...................... -

THE SYNTHESIS of SOME 1,2-Dn!ETHYL-3-;Jzyleenzq;ES Aim L,2,3-Trimirrhyl-Li
THE SYNTHESIS OF SOME 1,2-Dn!ETHYL-3-;JZYlEENZQ;ES Aim l,2,3-TRIMirrHYL-li-ALKYLBENZENES FROM 2-ALKÏLFURAKS AND 2-METHYL-5-AIi'DfLFURANS DISSERTATION Presented in Partial Fulfillment of the Requirements lo: the Degree of Doctor of Philosophy in the Graduate School of The Ohio State University By EARL PHILLIP MOORE, JR., B. S., W. S. The Ohio State University 1S?57 Approved by: Adviser Department r.f Chemistrj' ACKNOWLEDGiaæNT The author wishes to express his sincere appreciation to Professor Cecil E. Boord for his advice and counsel* Gratitude also is expressed to Dr. Kenneth W. Greenlee for his continued interest and guidance. Acknowledgement is made to Professor Boord and Dr. Greenlee for their cooperation in making available to the writer the equipment, materials and facilities of the American Petroleum Institute Research Project h$» The financial support of this work by the General Motors Corporation, E.I. du Pont de Nemours and Company, and the American Petroleum Institute Research Project LS is gratefully acknowledged. Table of Contents Page I. Introduction, 1 II. Literature Survey.......... ..... ..................... 3 A. l,2,3"Triinethylbenzene (Heraimellitene ) and l|2,3jl4“ Tetramethylbenzene (Prehnitene) from Natural Resources.............................................3 B. 1,2,3“Trimethylbenzene (Hemimellitene) from De gradations of Natural Products and their Derivatives & C. 1,2,3jh-Tetramethylbenzene (Prehnitene) from De gradations of Natural Products and their Derivatives I4 D. Syntheses of 1,2,3“Trimethylbenzene (Heraimellitene) and l,2,3,U“Tetramethylbenzene (Prehnitene).......... 5 E. The Synthesis of l,2-Diraethyl-3-8thylbenzene .... 10 III. Discussion............ ................................ 11 A. General Scope . 1 1 B. -

United States Patent L M116 “Patented Jan
‘I. i" a ‘l " 2,819,322 United States Patent l M116 “Patented Jan. 7., “1958 .11 .2 catalysts for the process, particularly‘the group VI metal sul?des, as, for example, the sul?des of tungsten, molyb ‘2,819,322 denum and chromium, and the sul?des of metals of atomic number from 25 to 28, especially nickel sul?de. PRODUCTION OF AROMATIC COMPOUNDS l ‘Composites of a metal sul?de from each of these. groups Lloyd C. Fetterly,”El'Cerrito, Cali?, a'ssigno'r to Shell Del are especially preferred. Corresponding oxides and com velopment Company, New York, N. Y., a corporation posites thereof are also suitable catalysts for'the'present of Delaware purpose, as well'as composites of oxides and sul?des. No Drawing. Application December '21, 1953 ‘ The diaryl methane compounds which are to be cracked 10 in accordance’with the invention, in generalare prepar Serial No. 399,570 able by the “alkylation” of the corresponding aryl com 9 Claims. (Cl. 260—668) pound with formaldehyde. ‘For example, it is known to prepare diphenylmethane, ditolylmethane " (mixture of isomers) and dixylylmethane, by reacting the correspond This invention relates to’ thepro'duction of methylaro 15' ing benzene/compound with formaldehyde in- the pres matic compounds,‘ and more'particularly to the produc ence of an acidic catalyst, such as sulfuric acid. _Simi tion of such‘compounds‘which contain a further alkyl larly, other compounds can be prepared,- such as di-beta radical, such as a second methyl radical, such as para 1 naphthylmethane from naphthalene, ~di§beta-5,6,7,8-tetra xylene, 2,6-dimethylnaphthalene and 'durene. -

Pages 23-Back Cover
STUDY UNIT DESIGN Study designs for both ground-water and surface- Surface-water studies focused on the unregulated por- water components focused principally on the Valley and tions of the Upper Tennessee River Basin principally in Ridge province. The Valley and Ridge is home to the the Valley and Ridge province, which contains the most majority of the Study Unit population and is the most intense agricultural activity in the basin. Thirteen basic highly developed in terms of agriculture and urban land fixed stream-sampling sites were operated during the uses. Ground-water studies focused on the carbonate- study to monitor water-quality conditions with time in based dolomites and limestones of the Valley and various parts of the basin. Data-collection sites were Ridge. These geologic units form the most prolific aqui- selected to cover the major subbasins of the Upper Ten- fers in the Upper Tennessee River Basin and also are the nessee River and to encompass the major land uses. An most susceptible to contamination because of their asso- additional 61 sites were sampled during the study as part ciated karst and solution features. Ground-water of three synoptic networks designed to better describe resources are very limited in the Blue Ridge and Cum- areal water-quality variations of the subbasins. In keep- berland Plateau provinces because of the relatively ing with the NAWQA multiple lines of evidence impermeable nature of the bedrock and the low water- approach to describe water-quality conditions,(34) data- storage capacity of the thin soils that overlie the bed- collection activities included water-column chemistry rock. -

United States Patent Office Patented Jan
3,636,177 United States Patent Office Patented Jan. 18, 1972 1. 2 it has been found that the disproportionation and trans 3,636,177 methylation reactions of tetramethylbenzenes proceed in PROCESS FOR PRODUCING DURENE Takashi Suzuki and Hiroyuki Iesaka, Niigata, Japan, quite different manners depending upon the type of assignors to Japan Gas Chemical Company, Inc., diluents used. Thus, in particular it was found that in Chiyoda-ku, Tokyo, Japan 5 producing durene by isomerization of isodurene, prehn No Drawing. Filed May 20, 1970, Ser. No. 39,145 itene, or mixtures of tetramethylbenzenes containing Claims priority, application Japan, May 22, 1969, durene in an amount less than that in the equilibrium com 44/39,121 position, it is possible to produce durene without decreas int, C. C07c5/28 ing the rate of isomerization, while effectively suppressing U.S. C. 260-668 A 7 Claims O the disproportionation or transmethylation reaction of tetramethylbenzenes by carrying out the isomerization in the presence of benzene or polymethylbenzenes having 9 ABSTRACT OF THE DISCLOSURE or less carbon atoms. Durene is produced in high yield by isomerizing other According to the present invention, durene is produced tetramethylbenzenes with a HF-BF catalyst in the by isomerization of isodurene, prehnitene, or mixtures of liquid phase in the presence of a diluent comprising ben tetramethylbenzenes in liquid phase in the presence of zene or methyl-substituted benzenes having 9 or less HF-BF3 catalyst. The isomerization is carried out in carbon atoms, whereby suppressing disproportionation and the presence of benzene or methyl-substituted benzenes transmethylation of tetramethylbenzenes. -

Heat Capacity of Liquids: Critical Review and Recommended Values
Heat Capacity of Liquids: Critical Review and Recommended Values. Supplement I Cite as: Journal of Physical and Chemical Reference Data 30, 1199 (2001); https:// doi.org/10.1063/1.1407866 Submitted: 20 February 2001 . Published Online: 10 January 2002 Milan Zábranský, Vlastimil Růžička, and Eugene S. Domalski ARTICLES YOU MAY BE INTERESTED IN Heat Capacity of Liquids: Critical Review and Recommended Values. Supplement II Journal of Physical and Chemical Reference Data 39, 013103 (2010); https:// doi.org/10.1063/1.3182831 Estimation of the Heat Capacities of Organic Liquids as a Function of Temperature using Group Additivity. II. Compounds of Carbon, Hydrogen, Halogens, Nitrogen, Oxygen, and Sulfur Journal of Physical and Chemical Reference Data 22, 619 (1993); https:// doi.org/10.1063/1.555924 Heat Capacities and Entropies of Organic Compounds in the Condensed Phase. Volume III Journal of Physical and Chemical Reference Data 25, 1 (1996); https://doi.org/10.1063/1.555985 Journal of Physical and Chemical Reference Data 30, 1199 (2001); https://doi.org/10.1063/1.1407866 30, 1199 © 2002 by the U.S. Secretary of Commerce on behalf of the United States. All rights reserved. Heat Capacity of Liquids: Critical Review and Recommended Values. Supplement I Milan Za´bransky´, Vlastimil Ru˚ zˇicˇka, Jr.,a… and Eugene S. Domalskib… Department of Physical Chemistry, Institute of Chemical Technology, 166 28 Prague 6, Czech Republic ͑Received 20 February 2001; revised manuscript received 30 July 2001͒ A study was carried out in which new experimental data on heat capacities of pure liquid organic and some inorganic compounds were compiled, critically evaluated, and recommended values provided. -

Acute Toxicity of Tetramethylbenzenes: Durene, Isodurene and Prehnitene
DRUG AND CHEMICAL TOXICOLOGY, 1(3), 219-230 (1978) ACUTE TOXICITY OF TETRAMETHYLBENZENES : DURENE, ISODURENE AND PREHNITENE Dennis ;J. Lynch Vernon R. Perone Ronald L. Schuler William B. Ushry Trent rl. Lewis 3epartment of Bealth, Education, and Welfare Public Health Service Center for Disease Control National Institute for Occupational Safety and Health 4676 Columbia Parkway Cincinnati, Ohio 45226 ABSTRACT Oral LD50 (rat), primary skin irritation (rabbit), cutaneous sensitization (guinea pig) and eye irritation (rabbit) studies For personal use only. were conducted on the three tetramethylbenzene isomers: durene, isodurene and prehnitene. The order of oral toxicity was isodurene > prehnitene > durene. Durene was not a skin irritant, while isodurene and prehnitene each produced a mild positive skin response (erythema). None of the tetramethylbenzenes were skin sensitizers or eye irritants. qurene, isodurene and prehnitene are only slightly toxic on an acute toxicologic basis and only pose an acute health hazard when injested in excessive quantities. Drug and Chemical Toxicology Downloaded from informahealthcare.com by Hacettepe Univ. on 11/24/12 219 Copyright 0 1978 hy Marcel Dekker, InL All Rights Reserved Neither this work nor dny pdrt may be reproduced or transmitted in dny form or by any means, electronic or mechanicdl including photocopying, microfilming, and recording, or by any information storage and retrieval system without permission in writing from the publisher 220 LYNCH ET AL. INTRODUCTION Durene (1,2,4,5-tetramethylbenzene), isodurene (1,2,3,5- tetramethylbenzene) and prehnitene (1,2,3,4-tetramethylbenzene) are used as starting materials in the organic synthesis of plastics and resins. At room temperature durene is a solid, while the other isomers are liquids. -

PCB and Environmental Neat Standards
PCB and Environmental Neat Standards Your essential resource for Agilent ULTRA chemical standards Table of contents Introduction 3 Aroclors 21 About Agilent standards 3 Commercial PCBs 21 Products 3 Markets 3 PCM, Metabolites, and Terphenyls 23 Custom products 3 Hydroxylated biphenyls 23 Quality control laboratory 4 Quality control validation levels 4 Polybrominated Biphenyls 24 Triple certification 5 Bromo biphenyls 24 Level 2 reference material Certificate of Analysis 6 Diphenyl Ethers 25 GHS compliance 7 Polychlorinated diphenyl ethers 25 Polychlorinated Biphenyls and Polybrominated diphenyl ethers 25 Environmental Neat Standards 8 Working with small quantities 8 Dioxins 26 PCB purities 8 Polychlorinated dibenzo-p-dioxins 26 BZ numbers 8 Dibenzofurans 28 PCB Congeners 9 Polychlorinated dibenzofurans 28 PCB congeners – neat and in solution 9 PCB congener mixtures 15 Additional Neat Compounds 29 Neat compounds of environmental interest 29 PCB Methods 17 EPA Method 680 17 Agilent Service and Support 39 EPA Method 8082 18 European PCB congener mixtures 19 PCB windowing mixtures 20 2 PCB and Environmental Neat Standards Introduction About Agilent standards Agilent is a global leader in chromatography and spectroscopy, as well as an expert in chemical standards manufacturing. Agilent offers certified reference materials, QC standards, reagents, and buffers to complement our extensive line of instruments, columns, sample preparation products, consumables, and services. Our portfolio provides laboratories with full workflow solutions for efficient, accurate results. Agilent has an extensive list of chemical standards, matched by expertise in designing and formulating custom standards to exacting specifications. Agilent products are available through our global distribution channels, and with our logistics capabilities we offer rapid turnaround time on all orders. -

United States Patent Office Paterated Jan
3,361,779 United States Patent Office Paterated Jan. 2, 1968 1. 2 bon of the aromatic ring is bonded apparently by co 3,361,779 ORGANOMETALLC COMPOUNDS ordinate covalence in a fashion such that the ring con Thomas H. Coffield, Heidelberg, Germany, and Rex D. tributes six electrons to the metal atom. Likewise, the Closson, Northville, Mich., assignors to Ethyl Corpo other electron donor groups also are covalently or co ration, New York, N.Y., a corporation of Virginia 5 ordinatively bonded to the metal atom. Such donation of No Drawing. Filed Aug. 20, 1959, Ser. No. 834,931 electrons contributes materially to the stability of the 18 Claims. (Cl. 260-429) molecule since the metal atoms, with the donated elec trons, approaches the electron configuration of the next This application is a continuation-in-part of my earlier higher rare gas. In a preferred embodiment of this inven filed copending application, U.S. Ser. No. 690,909, filed on () tion the compound has the electron configuration of the Oct. 18, 1957, now abandoned. next higher rare gas. For example, the chromium atom in This invention relates to novel organometallic com benzene chromium tricarbonyl has the electron configura pounds and their preparation. More particularly the pres tion of krypton. Thus, in the case of benzene chromium ent invention relates to novel and useful aromatic transi tricarbonyl, the three CO groups donate a total of six tion metal complexes. electrons and the benzene molecule donates six electrons, An object of this invention is to provide a novel class giving a stable compound which can be illustrated as foll of organometallic compounds.