Notes on the Ecology of the Little Bittern, Ixobrychus Minutus, At
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

La Mancha, Coto Donana & Extremadura 2017
Field Guides Tour Report Spain: La Mancha, Coto Donana & Extremadura 2017 May 6, 2017 to May 18, 2017 Chris Benesh & Godfried Schreur For our tour description, itinerary, past triplists, dates, fees, and more, please VISIT OUR TOUR PAGE. Spectacular skies greeted us during our visit to old Trujillo in the heart of Extremadura. Photo by guide Chris Benesh. So many birds around that you don´t know which to choose and observe. Do you recognize this feeling? We experienced many of these exciting moments in Spain during the Field Guides tour in May. It started straight away, on the first day, overlooking the natural lagoons of La Mancha Húmeda, where we had the chance to observe a great variety of species of ducks, grebes, terns, and passerines. The highlights here were the White-headed Duck, Eared Grebe, Red-crested Pochard, Whiskered Tern and Penduline Tit. In the National Park of Coto Donana again we found ourselves surrounded by birds: larks, bee-eaters, flamingos, Great Reed Warblers, Glossy Ibis, Squacco and Purple herons and a surprisingly well showing Little Bittern. With a bit of searching, scanning and listening we were able to also detect Red-knobbed Coot, Marbled Teal and Isabelline (Western Olivaceous) Warbler. Later in the week, close to Trujillo (Extremadura), we all enjoyed the excursion on the open, rolling plains, with Great and Little bustards, Eurasian Roller, Hoopoe, Calandra Lark, Montagu´s Harrier and many, many White Storks. For the shy Black Storks we had to wait one day more. In Monfrague National Park we discovered 3 pairs nesting on the breathtaking cliff of Peña Falcón. -

Australasian Bittern (Botaurus Poiciloptilus) Western Australian Recovery Plan
Australasian Bittern (Botaurus poiciloptilus) Western Australian Recovery Plan Wildlife Management Program No. 64 Western Australia Department of Biodiversity, Conservation and Attractions August 2018 Wildlife Management Program No. 64 Australasian Bittern (Botaurus poiciloptilus) Western Australian Recovery Plan August 2018 Western Australia Department of Biodiversity, Conservation and Attractions Locked Bag 104, Bentley Delivery Centre, Western Australia 6983 Foreword Recovery plans are developed within the framework provided in Department of Biodiversity, Conservation and Attractions Corporate Policy Statement No. 35 (Parks and Wildlife, 2015c), and the Australian Government Department of the Environment and Energy Recovery Planning Compliance Checklist for Legislative and Process Requirements (DoE, 2014). Recovery plans outline the recovery actions that are needed to urgently address those threatening processes most affecting the ongoing survival of threatened taxa or ecological communities, and begin the recovery process. The attainment of objectives and the provision of funds necessary to implement actions are subject to budgetary and other constraints affecting the parties involved, as well as the need to address other priorities. This plan will operate for a 10 year period but will remain in force until withdrawn or replaced and will be reviewed at least at five year intervals. This recovery plan was approved by the Department of Biodiversity, Conservation and Attractions, Western Australia. Approved recovery plans are subject -

Ixobrychus Minutus (Common Little Bittern)
Ixobrychus minutus (Common Little Bittern) European Red List of Birds Supplementary Material The European Union (EU27) Red List assessments were based principally on the official data reported by EU Member States to the European Commission under Article 12 of the Birds Directive in 2013-14. For the European Red List assessments, similar data were sourced from BirdLife Partners and other collaborating experts in other European countries and territories. For more information, see BirdLife International (2015). Contents Reported national population sizes and trends p. 2 Trend maps of reported national population data p. 4 Sources of reported national population data p. 6 Species factsheet bibliography p. 11 Recommended citation BirdLife International (2015) European Red List of Birds. Luxembourg: Office for Official Publications of the European Communities. Further information http://www.birdlife.org/datazone/info/euroredlist http://www.birdlife.org/europe-and-central-asia/european-red-list-birds-0 http://www.iucnredlist.org/initiatives/europe http://ec.europa.eu/environment/nature/conservation/species/redlist/ Data requests and feedback To request access to these data in electronic format, provide new information, correct any errors or provide feedback, please email [email protected]. THE IUCN RED LIST OF THREATENED SPECIES™ BirdLife International (2015) European Red List of Birds Ixobrychus minutus (Common Little Bittern) Table 1. Reported national breeding population size and trends in Europe1. Country (or Population estimate Short-term population trend4 Long-term population trend4 Subspecific population (where relevant) 2 territory) Size (pairs)3 Europe (%) Year(s) Quality Direction5 Magnitude (%)6 Year(s) Quality Direction5 Magnitude (%)6 Year(s) Quality Albania 100-350 <1 2002-2012 medium 0 0 2002-2012 medium - 10-20 1980-2012 poor Armenia 1,500-2,000 2 2002-2012 medium ? ? Austria 180-270 <1 2001-2012 medium 0 0 2001-2012 medium ? I. -

A Preliminary Risk Assessment of Cane Toads in Kakadu National Park Scientist Report 164, Supervising Scientist, Darwin NT
supervising scientist 164 report A preliminary risk assessment of cane toads in Kakadu National Park RA van Dam, DJ Walden & GW Begg supervising scientist national centre for tropical wetland research This report has been prepared by staff of the Environmental Research Institute of the Supervising Scientist (eriss) as part of our commitment to the National Centre for Tropical Wetland Research Rick A van Dam Environmental Research Institute of the Supervising Scientist, Locked Bag 2, Jabiru NT 0886, Australia (Present address: Sinclair Knight Merz, 100 Christie St, St Leonards NSW 2065, Australia) David J Walden Environmental Research Institute of the Supervising Scientist, GPO Box 461, Darwin NT 0801, Australia George W Begg Environmental Research Institute of the Supervising Scientist, GPO Box 461, Darwin NT 0801, Australia This report should be cited as follows: van Dam RA, Walden DJ & Begg GW 2002 A preliminary risk assessment of cane toads in Kakadu National Park Scientist Report 164, Supervising Scientist, Darwin NT The Supervising Scientist is part of Environment Australia, the environmental program of the Commonwealth Department of Environment and Heritage © Commonwealth of Australia 2002 Supervising Scientist Environment Australia GPO Box 461, Darwin NT 0801 Australia ISSN 1325-1554 ISBN 0 642 24370 0 This work is copyright Apart from any use as permitted under the Copyright Act 1968, no part may be reproduced by any process without prior written permission from the Supervising Scientist Requests and inquiries concerning reproduction -

Birds of Marakele National Park
BIRDS OF MARAKELE NATIONAL PARK English (Roberts 6) Old SA No. Rob No. English (Roberts 7) Global Names Names 1 1 Common Ostrich Ostrich 2 6 Great Crested Grebe Great Crested Grebe 3 8 Little Grebe Dabchick 4 50 Pinkbacked Pelican Pinkbacked Pelican 5 55 Whitebreasted Cormorant Whitebreasted Cormorant 6 58 Reed Cormorant Reed Cormorant 7 60 African Darter Darter 8 62 Grey Heron Grey Heron 9 63 Blackheaded Heron Blackheaded Heron 10 64 Goliath Heron Goliath Heron 11 65 Purple Heron Purple Heron 12 66 Great Egret Great White Egret 13 67 Little Egret Little Egret 14 68 Yellowbilled Egret Yellowbilled Egret 15 69 Black Heron Black Egret 16 71 Cattle Egret Cattle Egret 17 72 Squacco Heron Squacco Heron 18 74 Greenbacked Heron Greenbacked Heron 19 76 Blackcrowned Night-Heron Blackcrowned Night Heron 20 77 Whitebacked Night-Heron Whitebacked Night Heron 21 78 Little Bittern Little Bittern 22 79 Dwarf Bittern Dwarf Bittern 23 81 Hamerkop Hamerkop 24 83 White Stork White Stork 25 84 Black Stork Black Stork 26 85 Abdim's Stork Abdim's Stork 27 89 Marabou Stork Marabou Stork 28 90 Yellowbilled Stork Yellowbilled Stork 29 91 African Sacred Ibis Sacred Ibis 30 93 Glossy Ibis Glossy Ibis 31 94 Hadeda Ibis Hadeda Ibis 32 95 African Spoonbill African Spoonbill 33 96 Greater Flamingo Greater Flamingo 34 97 Lesser Flamingo Lesser Flamingo 35 99 Whitefaced Duck Whitefaced Duck 36 100 Fulvous Duck Fulvous Duck 37 101 Whitebacked Duck Whitebacked Duck 38 102 Egyptian Goose Egyptian Goose 39 103 South African Shelduck South African Shelduck 40 104 Yellowbilled -

D4.2.1 Review of the Knowledge of the Target Species at the Selected
ECOlogical observing System in the Adriatic Sea: oceanographic observations for biodiversity Priority Axis 3: Environment and cultural heritage Specific Objective 3.2: Contribute to protect and restore biodiversity D4.2.1 Review of the knowledge of the target species at the selected Natura 2000 sites WP4 – Establishing the Ecological Observing System in the Adriatic Sea (ECOAdS) A4.2 – Integration of ecological observing system with Natura 2000 target species PP5 Blue World Institute / Jure Miočić-Stošić, Grgur Pleslić Other involved partners: PP1, PP4, PP6, PP8 Final Public 29th June 2020 European Regional Development Fund www.italy-croatia.eu/ecoss Contents 1. INTRODUCTION ..................................................................................................................................... 4 2. ALGAE .................................................................................................................................................... 7 2.1 Fucus (Fucus virsoides) ........................................................................................................................ 7 3. PLANTS .................................................................................................................................................. 9 3.1 Virginia saltmarsh mallow (Kosteletzkya pentacarpos) ...................................................................... 9 3.2 Venice salicorne (Salicornia veneta) ................................................................................................. 10 -

EUROPEAN BIRDS of CONSERVATION CONCERN Populations, Trends and National Responsibilities
EUROPEAN BIRDS OF CONSERVATION CONCERN Populations, trends and national responsibilities COMPILED BY ANNA STANEVA AND IAN BURFIELD WITH SPONSORSHIP FROM CONTENTS Introduction 4 86 ITALY References 9 89 KOSOVO ALBANIA 10 92 LATVIA ANDORRA 14 95 LIECHTENSTEIN ARMENIA 16 97 LITHUANIA AUSTRIA 19 100 LUXEMBOURG AZERBAIJAN 22 102 MACEDONIA BELARUS 26 105 MALTA BELGIUM 29 107 MOLDOVA BOSNIA AND HERZEGOVINA 32 110 MONTENEGRO BULGARIA 35 113 NETHERLANDS CROATIA 39 116 NORWAY CYPRUS 42 119 POLAND CZECH REPUBLIC 45 122 PORTUGAL DENMARK 48 125 ROMANIA ESTONIA 51 128 RUSSIA BirdLife Europe and Central Asia is a partnership of 48 national conservation organisations and a leader in bird conservation. Our unique local to global FAROE ISLANDS DENMARK 54 132 SERBIA approach enables us to deliver high impact and long term conservation for the beneit of nature and people. BirdLife Europe and Central Asia is one of FINLAND 56 135 SLOVAKIA the six regional secretariats that compose BirdLife International. Based in Brus- sels, it supports the European and Central Asian Partnership and is present FRANCE 60 138 SLOVENIA in 47 countries including all EU Member States. With more than 4,100 staf in Europe, two million members and tens of thousands of skilled volunteers, GEORGIA 64 141 SPAIN BirdLife Europe and Central Asia, together with its national partners, owns or manages more than 6,000 nature sites totaling 320,000 hectares. GERMANY 67 145 SWEDEN GIBRALTAR UNITED KINGDOM 71 148 SWITZERLAND GREECE 72 151 TURKEY GREENLAND DENMARK 76 155 UKRAINE HUNGARY 78 159 UNITED KINGDOM ICELAND 81 162 European population sizes and trends STICHTING BIRDLIFE EUROPE GRATEFULLY ACKNOWLEDGES FINANCIAL SUPPORT FROM THE EUROPEAN COMMISSION. -

ABSTRACT Title of Dissertation: SECRETIVE MARSHBIRDS of URBAN WETLANDS in the WASHINGTON, DC METROPOLITAN AREA Patrice Nielson
ABSTRACT Title of Dissertation: SECRETIVE MARSHBIRDS OF URBAN WETLANDS IN THE WASHINGTON, DC METROPOLITAN AREA Patrice Nielson, Doctor of Philosophy 2016 Dissertation directed by: Dr. William Bowerman and Dr. Andrew Baldwin Environmental Science and Technology Secretive marshbirds are in decline across their range and are species of greatest conservation need in state Wildlife Action Plans. However, their secretive nature means there is relatively sparse information available on their ecology. There is demand for this information in the Washington, DC area for updating conservation plans and guiding wetland restoration. Rapid Wetland Assessment Methods are often used to monitor success of restoration but it is unknown how well they indicate marshbird habitat. Using the Standardized North American Marshbird Monitoring Protocol, I surveyed 51 points in 25 marshes in the DC area in 2013 – 2015. I also collected data on marsh area, buffer width, vegetation/water interspersion, vegetation characteristics, flooding, and invertebrates. At each bird survey point I assessed wetland quality using the Floristic Quality Assessment Index (FQAI) and California Rapid Wetland Assessment (CRAM) methods. I used Program Presence to model detection and occupancy probabilities of secretive marshbirds as a function of habitat variables. I found king rails (Rallus elegans) at five survey sites and least bittern (Ixobrychus exilis) at thirteen survey sites. Secretive marshbirds were using both restored and natural marshes, marshes with and without invasive plant species, and marshes with a variety of dominant vegetation species. King rail occupancy was positively correlated with plant diversity and invertebrate abundance and weakly negatively correlated with persistent vegetation. Least bittern occupancy was strongly negatively correlated woody vegetation and invertebrate abundance and weakly positively correlated with persistent vegetation. -

Springbirding in Spain: from the Atlantic Coast to The
SPRING BIRDING IN SPAIN: FROM THE ATLANTIC COAST TO THE HIGH PYRENEES APRIL 25-MAY 12, 2021 © 2020 VENT’s Spanish birding tour has been designed based on over 30 years of experience of birding in Spain. This itinerary incorporates important bird areas in Central and Northern Spain and additional important wetlands on the Atlantic coast. The route has been planned to add further exciting species and to make this an extremely comprehensive birding tour, with the possibility of including Middle Spotted Woodpecker, breeding Bluethroats and Rufous-tailed Scrub-Robin to an already very impressive tour list that stands at a total of 275 species and regularly exceeds 200 species. Spain has long been an extremely popular destination for European birders, offering as it does a wide range of typical Mediterranean habitats, along with easy, safe, and excellent birding. Many sites are in beautifully scenic settings featuring Romanesque architecture and ancient, fortified hilltop villages. Despite its appeal within Europe as a “sun and sand” holiday destination, much of interior Spain is very rural and way off the tourist trail. This is the finest region to sample a large array of southern European species and is especially good for raptors, with 24 species possible. Our very comprehensive itinerary is specially designed for American birders and provides a greater diversity of birds, concentrating on three major and distinct habitats: the semi-arid steppes and cork-oak dehesas of central Spain’s interior; the scenic sierras and high, alpine Pyrenees and Picos de Europa to the north in Aragon and Cantabria; and the Doñana wetlands and marshes on the Atlantic coast. -

Systematic List of the Romanian Vertebrate Fauna
Travaux du Muséum National d’Histoire Naturelle © Décembre Vol. LIII pp. 377–411 «Grigore Antipa» 2010 DOI: 10.2478/v10191-010-0028-1 SYSTEMATIC LIST OF THE ROMANIAN VERTEBRATE FAUNA DUMITRU MURARIU Abstract. Compiling different bibliographical sources, a total of 732 taxa of specific and subspecific order remained. It is about the six large vertebrate classes of Romanian fauna. The first class (Cyclostomata) is represented by only four species, and Pisces (here considered super-class) – by 184 taxa. The rest of 544 taxa belong to Tetrapoda super-class which includes the other four vertebrate classes: Amphibia (20 taxa); Reptilia (31); Aves (382) and Mammalia (110 taxa). Résumé. Cette contribution à la systématique des vertébrés de Roumanie s’adresse à tous ceux qui sont intéressés par la zoologie en général et par la classification de ce groupe en spécial. Elle représente le début d’une thème de confrontation des opinions des spécialistes du domaine, ayant pour but final d’offrir aux élèves, aux étudiants, aux professeurs de biologie ainsi qu’à tous ceux intéressés, une synthèse actualisée de la classification des vertébrés de Roumanie. En compilant différentes sources bibliographiques, on a retenu un total de plus de 732 taxons d’ordre spécifique et sous-spécifique. Il s’agît des six grandes classes de vertébrés. La première classe (Cyclostomata) est représentée dans la faune de Roumanie par quatre espèces, tandis que Pisces (considérée ici au niveau de surclasse) l’est par 184 taxons. Le reste de 544 taxons font partie d’une autre surclasse (Tetrapoda) qui réunit les autres quatre classes de vertébrés: Amphibia (20 taxons); Reptilia (31); Aves (382) et Mammalia (110 taxons). -
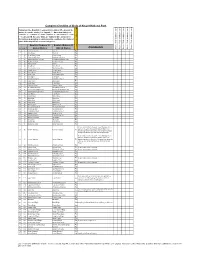
Kruger Comprehensive
Complete Checklist of birds of Kruger National Park Status key: R = Resident; S = present in summer; W = present in winter; E = erratic visitor; V = Vagrant; ? - Uncertain status; n = nomadic; c = common; f = fairly common; u = uncommon; r = rare; l = localised. NB. Because birds are highly mobile and prone to fluctuations depending on environmental conditions, the status of some birds may fall into several categories English (Roberts 7) English (Roberts 6) Comments Date of Trip and base camps Date of Trip and base camps Date of Trip and base camps Date of Trip and base camps Date of Trip and base camps # Rob # Global Names Old SA Names Rough Status of Bird in KNP 1 1 Common Ostrich Ostrich Ru 2 8 Little Grebe Dabchick Ru 3 49 Great White Pelican White Pelican Eu 4 50 Pinkbacked Pelican Pinkbacked Pelican Er 5 55 Whitebreasted Cormorant Whitebreasted Cormorant Ru 6 58 Reed Cormorant Reed Cormorant Rc 7 60 African Darter Darter Rc 8 62 Grey Heron Grey Heron Rc 9 63 Blackheaded Heron Blackheaded Heron Ru 10 64 Goliath Heron Goliath Heron Rf 11 65 Purple Heron Purple Heron Ru 12 66 Great Egret Great White Egret Rc 13 67 Little Egret Little Egret Rf 14 68 Yellowbilled Egret Yellowbilled Egret Er 15 69 Black Heron Black Egret Er 16 71 Cattle Egret Cattle Egret Ru 17 72 Squacco Heron Squacco Heron Ru 18 74 Greenbacked Heron Greenbacked Heron Rc 19 76 Blackcrowned Night-Heron Blackcrowned Night Heron Ru 20 77 Whitebacked Night-Heron Whitebacked Night Heron Ru 21 78 Little Bittern Little Bittern Eu 22 79 Dwarf Bittern Dwarf Bittern Sr 23 81 Hamerkop -

Comparative Study of Terrestrial Birds in the Disturbed and Undisturbed Vegetation Types of Ikere Forest Reserve, Ekiti State, Nigeria
Journal of Ecology and Natural Resources ISSN: 2578-4994 MEDWIN PUBLISHERS Committed to Create Value for researchers Comparative Study of Terrestrial Birds in the Disturbed and Undisturbed Vegetation Types of Ikere Forest Reserve, Ekiti State, Nigeria Ogunyemi OO* Department of Forest Resources and Wildlife management, Ekiti State University, Nigeria Review Article Volume 4 Issue 4 March 23, 2020 *Corresponding author: Ogunyemi OO, Department of Forest Resources and Wildlife Received Date: management, Ekiti State University, Ado-Ekiti, Nigeria, Email: olumideogunyemi80@yahoo. Published Date: May 21, 2020 com DOI: 10.23880/jenr-16000201 Abstract Birds are an important component of earth's ecosystems. The primary objective of the study was to assess the effect of Forest Reserve from January to December, 2017. Data were collected by employing transect count technique in the early disturbance on bird species diversity and abundance in the two stratified habitat types (disturbed and undisturbed) of Ikere types. During the study, a total of 551 individual birds comprising of 59 species, 18 families and 11 Orders were recorded. morning (6.00- 10.00a.m) and late afternoon (4.00- 6.00 p.m) for four consecutive days every month in the two vegetation recorded in the undisturbed vegetation. Order Passeriformes constituted the numerically dominant Order represented with Out of 59 identified species of birds, 46 (78%) of the species were recorded from disturbed vegetation, while 30 species were 16 species while Pelecaniformes, Galliformes and Colliformes were the least dominant Orders represented with one species each. At the family level, family Nectariniidae was numerically the dominant family represented with 9 species.