Recovery Plan for the Ocelot (Leopardus Pardalis) First Revision
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Grade 5 Curriculum Overview SS
Social Studies: 5th Grade Curriculum Overview North American Geography OVERVIEW: The standards in fifth grade focus student learning on the geography of North America. The introductory unit is designed to familiarize students with the many different tools and themes that geographers use to study the world. Students are introduced to some of the unique physical characteristics of the continents as they create their mental maps of the world. The skills and concepts introduced will continue to be practiced and refined as students study North America. Using these skills and the five 5th themes of geography, students will study five United States regions, Canada, Mexico, Central America, and the Caribbean Islands. Students will be able to compare and contrast the physical, cultural, and economic geography of regions and countries in North America. Sites that represent the cultural characteristics of each region provide students with a sense of what life in the region is like today. Regional Studies Essential Skills StudentsNAG will practice 5.3-5.9 and apply the following skills throughout the course: • Interpret a map legend (key) and a map scale to help understand information contained on a map. •The studentAnalyze will and explore interpret maps. •regionsAnalyze of the andUnited interpret graphs, charts, tables, and images of geographic information. •StatesInterpret and countries a model of of the earth showing the locations of the continents, oceans, equator, and prime meridian. •North AmericaDescribe theby: location of a continent by determining the hemisphere(s) where it is located. • Explain the use of GPS. •a. locatingCompare the regionand contrast on places using the five themes of geography. -

What Works in Conservation
What Works in Conservation 2017 EDITED BY WILLIAM J. SUTHERLAND, LYNN V. DICKS, NANCY OCKENDON AND REBECCA K. SMITH To access digital resources including: blog posts videos online appendices and to purchase copies of this book in: hardback paperback ebook editions Go to: https://www.openbookpublishers.com/product/552 Open Book Publishers is a non-profit independent initiative. We rely on sales and donations to continue publishing high-quality academic works. What Works in Conservation 2017 Edited by William J. Sutherland, Lynn V. Dicks, Nancy Ockendon and Rebecca K. Smith http://www.openbookpublishers.com © 2017 William J. Sutherland This work is licensed under a Creative Commons Attribution 4.0 International license (CC BY 4.0). This license allows you to share, copy, distribute and transmit the work; to adapt the work and to make commercial use of the work providing attribution is made to the authors (but not in any way that suggests that they endorse you or your use of the work). Attribution should include the following information: Sutherland, W.J., Dicks, L.V., Ockendon, N., and Smith, R.K. What Works in Conservation 2017. Cambridge, UK: Open Book Publishers, 2017. http://dx.doi.org/10.11647/OBP.0109 In order to access detailed and updated information on the license, please visit http://www.openbookpublishers.com/isbn/9781783743087#copyright Further details about CC BY licenses are available at http://creativecommons.org/licenses/by/4.0/ All links were active at the time of publication unless otherwise stated. Digital material and resources associated with this volume are available at http://www.openbookpublishers.com/isbn/9781783743087#resources and http://www.conservationevidence.com ISSN 2059-4232 (Print) ISSN 2059-4240 (Online) ISBN Paperback: 978-1-78374-308-7 ISBN Hardback: 978-1-78374-309-4 ISBN Digital (PDF): 978-1-78374-310-0 ISBN Digital ebook (epub): 978-1-78374-311-7 ISBN Digital ebook (mobi): 978-1-78374-312-4 DOI: 10.11647/OBP.0109 Funded by Arcadia, Synchronicity Earth, ESRC, NERC, Natural England and Waitrose Ltd. -

Fur Trade and the Biotic Homogenization of Subpolar Ecosystems
Chapter 14 Fur Trade and the Biotic Homogenization of Subpolar Ecosystems Ramiro D. Crego, Ricardo Rozzi, and Jaime E. Jiménez Abstract At the southern end of the Americas exist one of the last pristine ecosys- tems in the world, the sub-Antarctic Magellanic forests ecoregion, protected by the Cape Horn Biosphere Reserve (CHBR). Despite its remote location, the CHBR has been subject to the growing infuences of globalization, a process that has driven cultural, biotic, and economic transformations in the region since the mid-twentieth century. One of the most important threats to these unique ecosystems is the increase of biological invasions. Motivated by the expanding fur industry that responded to the globalization process, American beavers (Castor canadensis), muskrats (Ondatra zibethicus), and American minks (Neovison vison) were introduced, inde- pendently, to the southern tip of South America. Research has shown that these three North American species have reassembled their native interactions to affect negatively the invaded ecosystems of the CHBR. Beavers affect river fow and R. D. Crego (*) Department of Biological Sciences, University of North Texas, Denton, TX, USA Instituto de Ecología y Biodiversidad, Santiago, Chile Sub-Antarctic Biocultural Conservation Program, University of North Texas, Denton, TX, USA R. Rozzi Department of Philosophy and Religion and Department of Biological Sciences, University of North Texas, Denton, TX, USA Sub-Antarctic Biocultural Conservation Program, University of North Texas, Denton, TX, USA Instituto de Ecología y Biodiversidad and Universidad de Magallanes, Punta Arenas, Chile J. E. Jiménez Department of Biological Sciences, University of North Texas, Denton, TX, USA Instituto de Ecología y Biodiversidad, Santiago, Chile Sub-Antarctic Biocultural Conservation Program, University of North Texas, Denton, TX, USA Department of Philosophy and Religion, University of North Texas, Denton, TX, USA Universidad de Magallanes, Punta Arenas, Chile © Springer Nature Switzerland AG 2018 233 R. -

Oncilla 1 Oncilla
Oncilla 1 Oncilla Tiger Cat redirects here. For the Tom and Jerry Tales episode, see Tiger Cat (Tom and Jerry Tales). Oncilla[1] Conservation status [2] Vulnerable (IUCN 3.1) Scientific classification Kingdom: Animalia Phylum: Chordata Class: Mammalia Order: Carnivora Family: Felidae Genus: Leopardus Species: L. tigrinus Binomial name Leopardus tigrinus (Schreber, 1775) Oncilla 2 Oncilla range Synonyms Oncifelis tigrinus Felis tigrina The Oncilla (Leopardus tigrinus), also known as the Little Spotted Cat, Tigrillo, Cunaguaro or Tiger Cat, is a small spotted felid found in the tropical rainforests of Central and South America. It is a close relative of the Ocelot and the Margay, and has a rich ochre coat, spotted with black rosettes. The Oncilla is a nocturnal animal that hunts rodents and birds.[3] Appearance The Oncilla resembles the Margay and the Ocelot,[4] but is smaller, with a slender build and narrower muzzle. It grows to 38 to 59 centimetres (15 to 23 in) long, plus a 20 to 42 centimetres (7.9 to 17 in) tail.[5] While this is somewhat longer than the average domestic cat, Leopardus tigrinus is generally lighter, weighing 1.5 to 3 kilograms (3.3 to 6.6 lb).[6] The fur is thick and soft, ranging from light brown to dark ochre, with numerous dark rosettes across the back and flanks. The underside is pale with dark spots and the tail is ringed. The backs of the ears are black with bold ocelli.[4] The rosettes are black or brown, open in the center, and irregularly shaped.[7] The legs have medium-sized spots tapering to smaller spots near the paws.[7] This coloration helps the oncilla blend in with the mottled sunlight of the tropical forest understory. -

The Disastrous Impacts of Trump's Border Wall on Wildlife
a Wall in the Wild The Disastrous Impacts of Trump’s Border Wall on Wildlife Noah Greenwald, Brian Segee, Tierra Curry and Curt Bradley Center for Biological Diversity, May 2017 Saving Life on Earth Executive Summary rump’s border wall will be a deathblow to already endangered animals on both sides of the U.S.-Mexico border. This report examines the impacts of construction of that wall on threatened and endangered species along the entirety of the nearly 2,000 miles of the border between the United States and Mexico. TThe wall and concurrent border-enforcement activities are a serious human-rights disaster, but the wall will also have severe impacts on wildlife and the environment, leading to direct and indirect habitat destruction. A wall will block movement of many wildlife species, precluding genetic exchange, population rescue and movement of species in response to climate change. This may very well lead to the extinction of the jaguar, ocelot, cactus ferruginous pygmy owl and other species in the United States. To assess the impacts of the wall on imperiled species, we identified all species protected as threatened or endangered under the Endangered Species Act, or under consideration for such protection by the U.S. Fish and Wildlife Service (“candidates”), that have ranges near or crossing the border. We also determined whether any of these species have designated “critical habitat” on the border in the United States. Finally, we reviewed available literature on the impacts of the existing border wall. We found that the border wall will have disastrous impacts on our most vulnerable wildlife, including: 93 threatened, endangered and candidate species would potentially be affected by construction of a wall and related infrastructure spanning the entirety of the border, including jaguars, Mexican gray wolves and Quino checkerspot butterflies. -

Costa Rica: National Parks & Tropical Forests January 19 - 31, 2019 (13 Days) with Hamilton Professor of Biology Emeritus Dr
Costa Rica: National Parks & Tropical Forests January 19 - 31, 2019 (13 Days) with Hamilton Professor of Biology Emeritus Dr. Ernest H. Williams An exclusive Hamilton Global Adventure for 16 alumni, parents, and friends. © by Don Mezzi © T R Shankar Raman © by Steve © by Lars0001 3 San Carlos Rio Frio Costa Rica Altamira Village Dear Hamilton Alumni, Parents, and Friends, Lake Arenal I am delighted to invite you to join me in January 2019 for Monteverde Tortuguero 3 Cloud Forest National Park a wonderful trip to Costa Rica. As we travel from volcanic Reserve Doka Estate mountain ranges to misty cloud forests and bountiful jungles, San José our small group of no more than sixteen travelers, plus an Hacienda 2 Nosavar Santa Ana expert local Trip Leader and me, will explore these habitats up- close. Quepos San Gerardo 2 The biodiversity found in Costa Rica is astonishing for a country with Manuel de Dota 2 Antonio an area of just 20,000 square miles (approximately four times the size of National Park Finca don Connecticut): more than 12,000 species of plants, including a dazzling variety Tavo of trees and orchids; 237 species of mammals, including jaguars and four Main Tour species of monkeys; more species of birds (800!) than in all of North America; Optional Extensions more species of butterflies than on the entire continent of Africa; and five # of Hotel Nights genera of sea turtles as well as the endangered American crocodile. Corcovado Airport Arrival/ National Park Our travels will merge daily nature observations with visits to Costa Rican Departure national parks, farms, villages, beaches, cloud forest, and the capital city, San Jose. -

OCELOT CLUB Volume 18, Number 1454 Fleetwood Drive East January - February 1974 Mobile, Alabama 36605 OCELOT CLUB
Contents: Yukon Lynx Man ....................... Page 3 Pacific Northwest Report. ............ Page 4 Bengals, Bottles & Bleed II...... ....Page 5 Endangered Species Act. .............. Page 7 Readers Write. .......................Page 9 Greater New York Report.. ............Page 10 President's Statement. ............... Page 11 "El Tigre ............................. Page 12 Cougar Country ....................... Page 13 Florida Report ....................... Page 15 I- I LONG ISLAND OCELOT CLUB volume 18, Number 1454 Fleetwood Drive East January - February 1974 Mobile, Alabama 36605 OCELOT CLUB SULTAN, with Dave Salisbury of Cocoa, Florida. Our main interest was to see if it was possible to take a big cat, raise it as a loving pet and train it to perform. We've spent 14 months training Sultan to perform in our own act. Sultan is now four years old and weighs 143 pounds. Dave uses only the reward system to train Sultan and says that with Sultan's love of beef you need only to convey to him what has to be done in order to obtain the reward. Sultan has appeared in Life Magazine and on What's My Line? television show. Look for Sultan in the June or July issue of Playboy where he appears attired in hi-s sleek black coat. His co-star Miss Helen Rooney appears in a necklace. Dave is past President of the Florida Chapter and received a Lotty in 1969 for his work in LIOC. Branch Representatives: A. C. E. C. - Mrs. Ginny Story, 2475 Las Palomas, La Habra Heights, California 90631 CANADA - Mrs. Janet Thomas R.R.1, Box 602, Manotick, Ontario, (613j 692-4095 692-3728 CANADA - WEST- Doug Fletcher, 11431 73rd Ave., Delta, B.C., Canada Coordinator - Evelyn Dyck, 4911 Union St., North Burnaby, B.C. -

IUCN World Conservation Congress
S IUCN Resolutions, Recommendations and other Decisions World Conservation Congress Honolulu, Hawai‘i, United States of America 6 –10 September 2016 IUCN Resolutions, Recommendations and other Decisions World Conservation Congress Honolulu, Hawai‘i, United States of America 6–10 September 2016 The designation of geographical entities in this book, and the presentation of the material, do not imply the expression of any opinion whatsoever on the part of IUCN concerning the legal status of any country, territory, or area, or of its authorities, or concerning the delimitation of its frontiers or boundaries. The views expressed in this publication do not necessarily reflect those of IUCN. Published by: IUCN, Gland, Switzerland Copyright: © 2016 International Union for Conservation of Nature and Natural Resources Reproduction of this publication for educational or other non-commercial purposes is authorised without prior written permission from the copyright holder provided the source is fully acknowledged. Reproduction of this publication for resale or other commercial purposes is prohibited without prior written permission of the copyright holder. Citation: IUCN (2016). IUCN Resolutions, Recommendations and other Decisions. Gland, Switzerland: IUCN. 106pp. Produced by: IUCN Publications Unit Available for download from: www.iucn.org/resources/publications Contents Foreword 5 Acknowledgements 8 Table of Resolutions, Recommendations and other Decisions 10 Resolutions 17 Recommendations 216 Other Decisions 244 Annex 1 – Explanation of votes 248 Annex 2 – Statement of the United States Government on the IUCN Motions Process 297 3 Foreword It is with great pleasure that the Resolutions Committee hereby forwards to IUCN Members, Commission members, IUCN Secretariat staff, other Congress participants and all interested parties, the Resolutions and Recommendations as well as other key decisions adopted by the Members’ Assembly at the World Conservation Congress held in Hawai‘i, United States of America, 1–10 September 2016. -
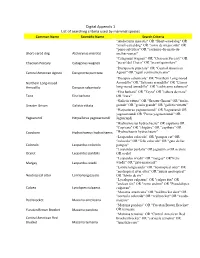
Digital Appendix 1 List of Searching Criteria Used by Mammal Species
Digital Appendix 1 List of searching criteria used by mammal species Common Name Scientific Name Search Criteria "Atelocynus microtis" OR "Short-eared dog" OR "small-eared dog" OR "zorro de oreja corta" OR "perro selvático" OR "cachorro-do-mato-de- Short-eared dog Atelocynus microtis orelhas-curtas" "Catagonus wagneri" OR "Chacoan Peccary" OR Chacoan Peccary Catagonus wagneri "pecarí del Chaco" OR "pecarí quimilero" “Dasyprocta punctate” OR "Central American Central American Agouti Dasyprocta punctata Agouti" OR "agutí centroamericano" “Dasypus sabanicola” OR "Northern Long-nosed Northern Long-nosed Armadillo" OR "Savanna armadillo" OR "Llanos Armadillo Dasypus sabanicola long-nosed armadillo" OR "cachicamo sabanero" “Eira barbara” OR "Tayra" OR "cabeza de mate" Taira Eira barbara OR "irara" “Galictis vittata” OR "Greater Grison" OR "furão- Greater Grison Galictis vittata grande" OR "grisón grande" OR "galictis vittatta" “Herpailurus yagouaroundi” OR Yaguarundi OR yagouaroundi OR "Puma yagouaroundi" OR Yaguarundi Herpailurus yagouaroundi Jaguarundi "Hydrochoerus hydrochaeris" OR capybara OR "Capivara" OR "chigüire" OR "capibara" OR Capybara Hydrochoerus hydrochaeris "Hydrochaeris hydrochaeris" “Leopardus colocolo” OR "pampas cat" OR "colocolo" OR "felis colocolo" OR "gato de las Colocolo Leopardus colocolo pampas" "Leopardus pardalis" OR jaguatirica OR ocelote Ocelot Leopardus pardalis OR ocelot “Leopardus wiedii” OR "margay" OR"Felis Margay Leopardus wiedii wiedii" OR "gato-maracajá" “Lontra longicaudis” OR "neotropical otter" OR "neotropical -

Spring in South Texas
SPRING IN SOUTH TEXAS MARCH 31–APRIL 9, 2019 Green Jay, Quinta Mazatlan, McAllen, Texas, April 5, 2019, Barry Zimmer LEADERS: BARRY ZIMMER & JACOB DRUCKER LIST COMPILED BY: BARRY ZIMMER VICTOR EMANUEL NATURE TOURS, INC. 2525 WALLINGWOOD DRIVE, SUITE 1003 AUSTIN, TEXAS 78746 WWW.VENTBIRD.COM SPRING IN SOUTH TEXAS MARCH 31–APRIL 9, 2019 By Barry Zimmer Once again, our Spring in South Texas tour had it all—virtually every South Texas specialty, wintering Whooping Cranes, plentiful migrants (both passerine and non- passerine), and rarities on several fronts. Our tour began with a brief outing to Tule Lake in north Corpus Christi prior to our first dinner. Almost immediately, we were met with a dozen or so Scissor-tailed Flycatchers lining a fence en route—what a welcoming party! Roseate Spoonbill, Crested Caracara, a very cooperative Long-billed Thrasher, and a group of close Cave Swallows rounded out the highlights. Strong north winds and unsettled weather throughout that day led us to believe that we might be in for big things ahead. The following day was indeed eventful. Although we had no big fallout in terms of numbers of individuals, the variety was excellent. Scouring migrant traps, bays, estuaries, coastal dunes, and other habitats, we tallied an astounding 133 species for the day. A dozen species of warblers included a stunningly yellow male Prothonotary, a very rare Prairie that foraged literally at our feet, two Yellow-throateds at arm’s-length, four Hooded Warblers, and 15 Northern Parulas among others. Tired of fighting headwinds, these birds barely acknowledged our presence, allowing unsurpassed studies. -

Carbon Offsetting Project in Brazil
Preserving native forests Since 2010, Atos has supported its customers in their journey towards more sustainable operations and has off set each year the total carbon emissions of all its data centers. In 2018, Atos has expanded this program to cover 100% of residual emissions of its data centers, offi ces, and business trips. In 2019, in partnership with EcoAct, 242,986 tCO2e were thus compensated. Thanks to a new investment made in 2020, Atos has enlarged its existing support to renewable energies to carbon sink preservation projects. An important development for the preservation of the climate. Among the projects supported, Atos invested in a preservation project of native forests in Brazil, as climate-science and market trends have demonstrated the importance of preserving and developing carbon sinks like forests or mangroves. A key leverage to ensure GHG emissions are still sequestrated or captured, in addition to usual emissions reductions and contributing to UN Sustainable Development Goals 8,12, 13 and 15. Project The project aims to protect the fragile environment of the Portel region in Brazil by preventing unplanned deforestation through the implementation of a land management system. It combines a rigorous monitoring of the area and an enforcement plan managed by local villagers trained to forest management and monitoring techniques. The villagers are therefore in charge of identifying and removing illegal activities such as logging, squattering and attempts to implement pastures or cattle ranching. The Project also provides capacity building on agroforestry systems and distribution of energy eff icient cook stoves for cassava production, hence reducing even more the local deforestation and developing new sources of Project Floresta de Portel © EcoAct, RMDLT revenues to local population. -

THE CASE AGAINST Marine Mammals in Captivity Authors: Naomi A
s l a m m a y t T i M S N v I i A e G t A n i p E S r a A C a C E H n T M i THE CASE AGAINST Marine Mammals in Captivity The Humane Society of the United State s/ World Society for the Protection of Animals 2009 1 1 1 2 0 A M , n o t s o g B r o . 1 a 0 s 2 u - e a t i p s u S w , t e e r t S h t u o S 9 8 THE CASE AGAINST Marine Mammals in Captivity Authors: Naomi A. Rose, E.C.M. Parsons, and Richard Farinato, 4th edition Editors: Naomi A. Rose and Debra Firmani, 4th edition ©2009 The Humane Society of the United States and the World Society for the Protection of Animals. All rights reserved. ©2008 The HSUS. All rights reserved. Printed on recycled paper, acid free and elemental chlorine free, with soy-based ink. Cover: ©iStockphoto.com/Ying Ying Wong Overview n the debate over marine mammals in captivity, the of the natural environment. The truth is that marine mammals have evolved physically and behaviorally to survive these rigors. public display industry maintains that marine mammal For example, nearly every kind of marine mammal, from sea lion Iexhibits serve a valuable conservation function, people to dolphin, travels large distances daily in a search for food. In learn important information from seeing live animals, and captivity, natural feeding and foraging patterns are completely lost.