AP42 Chapter 9 Reference
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Development and Validation of Liquid Chromatography-Based Methods to Assess the Lipophilicity of Cytotoxic Platinum(IV) Complexes
Article Development and Validation of Liquid Chromatography-Based Methods to Assess the Lipophilicity of Cytotoxic Platinum(IV) Complexes Matthias H. M. Klose 1,2, Sarah Theiner 3, Hristo P. Varbanov 1,4, Doris Hoefer 1, Verena Pichler 1,5, Markus Galanski 1, Samuel M. Meier-Menches 2,3,* and Bernhard K. Keppler 1,2,* 1 Institute of Inorganic Chemistry, University of Vienna, 1090 Vienna, Austria; [email protected] (M.H.M.K.); [email protected] (D.H.); [email protected] (M.G.) 2 Research Cluster ‘Translational Cancer Therapy Research’, University of Vienna, 1090 Vienna, Austria 3 Department of Analytical Chemistry, University of Vienna, 1090 Vienna, Austria; [email protected] 4 Institute of Chemistry—Inorganic Chemistry, University of Graz, 8010 Graz, Austria; [email protected] (H.P.V.) 5 Division of Nuclear Medicine, Department of Biomedical Imaging and Image-Guided Therapy, Medical University of Vienna, 1090 Vienna, Austria; [email protected] (V.P.) * Correspondence: [email protected] (S.M.M.-M.); [email protected] (B.K.K.); Tel.: +43-1-4277-52373 (S.M.M.-M.); +43-1-4277-52602 (B.K.K.) Received: 2 October 2018; Accepted: 29 November 2018; Published: 4 December 2018 Abstract: Lipophilicity is a crucial parameter for drug discovery, usually determined by the logarithmic partition coefficient (Log P) between octanol and water. However, the available detection methods have restricted the widespread use of the partition coefficient in inorganic medicinal chemistry, and recent investigations have shifted towards chromatographic lipophilicity parameters, frequently without a conversion to derive Log P. -

Liquid-Liquid Extraction
OCTOBER 2020 www.processingmagazine.com A BASIC PRIMER ON LIQUID-LIQUID EXTRACTION IMPROVING RELIABILITY IN CHEMICAL PROCESSING WITH PREVENTIVE MAINTENANCE DRIVING PACKAGING SUSTAINABILITY IN THE TIME OF COVID-19 Detecting & Preventing Pressure Gauge AUTOMATIC RECIRCULATION VALVES Failures Schroedahl www.circor.com/schroedahl page 48 LOW-PROFILE, HIGH-CAPACITY SCREENER Kason Corporation www.kason.com page 16 chemical processing A basic primer on liquid-liquid extraction An introduction to LLE and agitated LLE columns | By Don Glatz and Brendan Cross, Koch Modular hemical engineers are often faced with The basics of liquid-liquid extraction the task to design challenging separation While distillation drives the separation of chemicals C processes for product recovery or puri- based upon dif erences in relative volatility, LLE is a fication. This article looks at the basics separation technology that exploits the dif erences in of one powerful and yet overlooked separation tech- the relative solubilities of compounds in two immis- nique: liquid-liquid extraction. h ere are other unit cible liquids. Typically, one liquid is aqueous, and the operations used to separate compounds, such as other liquid is an organic compound. distillation, which is taught extensively in chemical Used in multiple industries including chemical, engineering curriculums. If a separation is feasible by pharmaceutical, petrochemical, biobased chemicals distillation and is economical, there is no reason to and l avor and fragrances, this approach takes careful consider liquid-liquid extraction (LLE). However, dis- process design by experienced chemical engineers and tillation may not be a feasible solution for a number scientists. In many cases, LLE is the best choice as a of reasons, such as: separation technology and well worth searching for a • If it requires a complex process sequence (several quali ed team to assist in its development and design. -

Selection of Thermodynamic Methods
P & I Design Ltd Process Instrumentation Consultancy & Design 2 Reed Street, Gladstone Industrial Estate, Thornaby, TS17 7AF, United Kingdom. Tel. +44 (0) 1642 617444 Fax. +44 (0) 1642 616447 Web Site: www.pidesign.co.uk PROCESS MODELLING SELECTION OF THERMODYNAMIC METHODS by John E. Edwards [email protected] MNL031B 10/08 PAGE 1 OF 38 Process Modelling Selection of Thermodynamic Methods Contents 1.0 Introduction 2.0 Thermodynamic Fundamentals 2.1 Thermodynamic Energies 2.2 Gibbs Phase Rule 2.3 Enthalpy 2.4 Thermodynamics of Real Processes 3.0 System Phases 3.1 Single Phase Gas 3.2 Liquid Phase 3.3 Vapour liquid equilibrium 4.0 Chemical Reactions 4.1 Reaction Chemistry 4.2 Reaction Chemistry Applied 5.0 Summary Appendices I Enthalpy Calculations in CHEMCAD II Thermodynamic Model Synopsis – Vapor Liquid Equilibrium III Thermodynamic Model Selection – Application Tables IV K Model – Henry’s Law Review V Inert Gases and Infinitely Dilute Solutions VI Post Combustion Carbon Capture Thermodynamics VII Thermodynamic Guidance Note VIII Prediction of Physical Properties Figures 1 Ideal Solution Txy Diagram 2 Enthalpy Isobar 3 Thermodynamic Phases 4 van der Waals Equation of State 5 Relative Volatility in VLE Diagram 6 Azeotrope γ Value in VLE Diagram 7 VLE Diagram and Convergence Effects 8 CHEMCAD K and H Values Wizard 9 Thermodynamic Model Decision Tree 10 K Value and Enthalpy Models Selection Basis PAGE 2 OF 38 MNL 031B Issued November 2008, Prepared by J.E.Edwards of P & I Design Ltd, Teesside, UK www.pidesign.co.uk Process Modelling Selection of Thermodynamic Methods References 1. -

Distillation Theory
Chapter 2 Distillation Theory by Ivar J. Halvorsen and Sigurd Skogestad Norwegian University of Science and Technology Department of Chemical Engineering 7491 Trondheim, Norway This is a revised version of an article published in the Encyclopedia of Separation Science by Aca- demic Press Ltd. (2000). The article gives some of the basics of distillation theory and its purpose is to provide basic understanding and some tools for simple hand calculations of distillation col- umns. The methods presented here can be used to obtain simple estimates and to check more rigorous computations. NTNU Dr. ing. Thesis 2001:43 Ivar J. Halvorsen 28 2.1 Introduction Distillation is a very old separation technology for separating liquid mixtures that can be traced back to the chemists in Alexandria in the first century A.D. Today distillation is the most important industrial separation technology. It is particu- larly well suited for high purity separations since any degree of separation can be obtained with a fixed energy consumption by increasing the number of equilib- rium stages. To describe the degree of separation between two components in a column or in a column section, we introduce the separation factor: ()⁄ xL xH S = ------------------------T (2.1) ()x ⁄ x L H B where x denotes mole fraction of a component, subscript L denotes light compo- nent, H heavy component, T denotes the top of the section, and B the bottom. It is relatively straightforward to derive models of distillation columns based on almost any degree of detail, and also to use such models to simulate the behaviour on a computer. -

Lipophilicity (Logd7.4) of N-Aryl Benzo Hydroxamic Acids
Global Journal of Pharmacy & Pharmaceutical Sciences ISSN: 2573-2250 Mini Review Glob J Pharmaceu Sci Volume 4 Issue 5 - february 2018 Copyright © All rights are reserved by Ajita Dixit DOI: 10.19080/GJPPS.2018.04.555648 Lipophilicity (LogD7.4) of N-Aryl Benzo Hydroxamic Acids Ajita Dixit* Pandit Ravishankar Shukla University, India Submission: July 24, 2017; Published: February 21, 2018 *Corresponding author: Ajita Dixit, Pandit Ravishankar Shukla University, India, Email: Abstract Hydroxamic acids are polyfunctional molecules which show a wide spectrum of biological and medicinal activities. Lipophilicity is known to be important for absorption, permeability and in vivo distribution of organic compound. Lipophilicity is also a major structural factor that influences the pharmacokinetic and pharmacodynamic behaviour of compound. Introduction homogeneously but rather, a gradient is formed that varies Lipophilicity is known to be important for absorption, with the composition and geometry of membrane [7]. The high permeability and in vivo distribution of organic compound degree of ordering of solutes in a lipid bi layer compared with a [1]. Since about one century, it is recognized as a meaningful parameter in Structure-activity relationship studies and with and partitioning. Never the less good correlation between the epoch making contributions of Hansch et al. [2] has become bulk liquid phase also significantly changes the thermodynamic the single most informative and successful physico-chemical membrane /buffer and octanol/water two phase’s systems has the partition coefficient of various lipophilic compounds in been observed [8]. property in medicinal chemistry. Lipophilicity is defined for a lipophilic environment.” Lipophilicity is determined Material and Methods “Lipophilicity represents the affinity of a molecule or a moiety valid only for a single chemical species) or as distribution experimentally as partition coefficients (written as logP and In the present investigation partition coefficient or n-Octanol and buffer as solvent. -

Distillation Accessscience from McgrawHill Education
6/19/2017 Distillation AccessScience from McGrawHill Education (http://www.accessscience.com/) Distillation Article by: King, C. Judson University of California, Berkeley, California. Last updated: 2014 DOI: https://doi.org/10.1036/10978542.201100 (https://doi.org/10.1036/10978542.201100) Content Hide Simple distillations Fractional distillation Vaporliquid equilibria Distillation pressure Molecular distillation Extractive and azeotropic distillation Enhancing energy efficiency Computational methods Stage efficiency Links to Primary Literature Additional Readings A method for separating homogeneous mixtures based upon equilibration of liquid and vapor phases. Substances that differ in volatility appear in different proportions in vapor and liquid phases at equilibrium with one another. Thus, vaporizing part of a volatile liquid produces vapor and liquid products that differ in composition. This outcome constitutes a separation among the components in the original liquid. Through appropriate configurations of repeated vaporliquid contactings, the degree of separation among components differing in volatility can be increased manyfold. See also: Phase equilibrium (/content/phaseequilibrium/505500) Distillation is by far the most common method of separation in the petroleum, natural gas, and petrochemical industries. Its many applications in other industries include air fractionation, solvent recovery and recycling, separation of light isotopes such as hydrogen and deuterium, and production of alcoholic beverages, flavors, fatty acids, and food oils. Simple distillations The two most elementary forms of distillation are a continuous equilibrium distillation and a simple batch distillation (Fig. 1). http://www.accessscience.com/content/distillation/201100 1/10 6/19/2017 Distillation AccessScience from McGrawHill Education Fig. 1 Simple distillations. (a) Continuous equilibrium distillation. -

Distillation Design the Mccabe-Thiele Method Distiller Diagam Introduction
Distillation Design The McCabe-Thiele Method Distiller diagam Introduction • UiUsing r igorous tray-bby-tray callilculations is time consuming, and is often unnecessary. • OikhdfiifbOne quick method of estimation for number of plates and feed stage can be obtained from the graphical McCabe-Thiele Method. • This eliminates the need for tedious calculations, and is also the first step to understanding the Fenske-Underwood- Gilliland method for multi-component distillation • Typi ica lly, the in le t flow to the dis tilla tion co lumn is known, as well as mole percentages to feed plate, because these would be specified by plant conditions. • The desired composition of the bottoms and distillate prodillbifiddhiilldducts will be specified, and the engineer will need to design a distillation column to produce these results. • With the McCabe-Thiele Method, the total number of necessary plates, as well as the feed plate location can biddbe estimated, and some ifiinformation can a lblso be determined about the enthalpic condition of the feed and reflux ratio. • This method assumes that the column is operated under constant pressure, and the constant molal overflow assumption is necessary, which states that the flow rates of liquid and vapor do not change throughout the column. • To understand this method, it is necessary to first elaborate on the subjects of the x-y diagram, and the operating lines used to create the McCabe-Thiele diagram. The x-y diagram • The x-y diagram depi icts vapor-liliqu id equilibrium data, where any point on the curve shows the variations of the amount of liquid that is in equilibrium with vapor at different temperatures. -
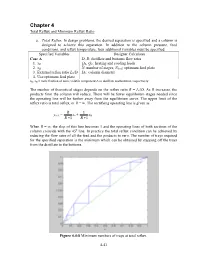
Minimum Reflux Ratio
Chapter 4 Total Reflux and Minimum Reflux Ratio a. Total Reflux . In design problems, the desired separation is specified and a column is designed to achieve this separation. In addition to the column pressure, feed conditions, and reflux temperature, four additional variables must be specified. Specified Variables Designer Calculates Case A D, B: distillate and bottoms flow rates 1. xD QR, QC: heating and cooling loads 2. xB N: number of stages, Nfeed : optimum feed plate 3. External reflux ratio L0/D DC: column diameter 4. Use optimum feed plate xD, xB = mole fraction of more volatile component A in distillate and bottoms, respectively The number of theoretical stages depends on the reflux ratio R = L0/D. As R increases, the products from the column will reduce. There will be fewer equilibrium stages needed since the operating line will be further away from the equilibrium curve. The upper limit of the reflux ratio is total reflux, or R = ∞. The rectifying operating line is given as R 1 yn+1 = xn + xD R +1 R +1 When R = ∞, the slop of this line becomes 1 and the operating lines of both sections of the column coincide with the 45 o line. In practice the total reflux condition can be achieved by reducing the flow rates of all the feed and the products to zero. The number of trays required for the specified separation is the minimum which can be obtained by stepping off the trays from the distillate to the bottoms. Figure 4.4-8 Minimum numbers of trays at total reflux. -

I. an Improved Procedure for Alkene Ozonolysis. II. Exploring a New Structural Paradigm for Peroxide Antimalarials
University of Nebraska - Lincoln DigitalCommons@University of Nebraska - Lincoln Student Research Projects, Dissertations, and Theses - Chemistry Department Chemistry, Department of 2011 I. An Improved Procedure for Alkene Ozonolysis. II. Exploring a New Structural Paradigm for Peroxide Antimalarials. Charles Edward Schiaffo University of Nebraska-Lincoln Follow this and additional works at: https://digitalcommons.unl.edu/chemistrydiss Part of the Organic Chemistry Commons Schiaffo, Charles Edward, "I. An Improved Procedure for Alkene Ozonolysis. II. Exploring a New Structural Paradigm for Peroxide Antimalarials." (2011). Student Research Projects, Dissertations, and Theses - Chemistry Department. 23. https://digitalcommons.unl.edu/chemistrydiss/23 This Article is brought to you for free and open access by the Chemistry, Department of at DigitalCommons@University of Nebraska - Lincoln. It has been accepted for inclusion in Student Research Projects, Dissertations, and Theses - Chemistry Department by an authorized administrator of DigitalCommons@University of Nebraska - Lincoln. I. An Improved Procedure for Alkene Ozonolysis. II. Exploring a New Structural Paradigm for Peroxide Antimalarials. By Charles E. Schiaffo A DISSERTATION Presented to the Faculty of The Graduate College at the University of Nebraska In Partial Fulfillment of Requirements For the Degree of Doctor of Philosophy Major: Chemistry Under the Supervision of Professor Patrick H. Dussault Lincoln, Nebraska June, 2011 I. An Improved Procedure for Alkene Ozonolysis. II. Exploring a New Structural Paradigm for Peroxide Antimalarials. Charles E. Schiaffo, Ph.D. University of Nebraska-Lincoln, 2011 Advisor: Patrick H. Dussault The use of ozone for the transformation of alkenes to carbonyls has been well established. The reaction of ozone with alkenes in this fashion generates either a 1,2,4- trioxolane (ozonide) or a hydroperoxyacetal, either of which must undergo a separate reduction step to provide the desired carbonyl compound. -

Lipophilicity As a Central Component of Drug-Like Properties of Chalchones and Flavonoid Derivatives
molecules Article Lipophilicity as a Central Component of Drug-Like Properties of Chalchones and Flavonoid Derivatives Teodora Constantinescu 1, Claudiu Nicolae Lungu 2,* and Ildiko Lung 3 1 Department of Chemistry, Faculty of Pharmacy, Iuliu Hatieganu University, 400012 Cluj-Napoca, Romania; [email protected] 2 Department of Chemistry, Faculty of Chemistry and Chemical Engineering, Babes-Bolyai University, 400028 Cluj-Napoca, Romania 3 National Institute for Research & Development of Isotopic and Molecular Technologies 67-103 Donath street, 400293 Cluj-Napoca, Romania; [email protected] * Correspondence: [email protected]; Tel.: +40-(0)-742-255-099 Received: 11 March 2019; Accepted: 10 April 2019; Published: 17 April 2019 Abstract: Lipophilcity is an important physico-chemical parameter that influences membrane transport and binding ability to action. Migration distance following complete elution of compounds was used to calculate different lipophilicity-related parameters. The aim of this study is to show that lipophilicity is a central component of thiazole chalcones and flavonoid derivatives regarding their drug-like properties. Experimental and computational methods were used. This study considers 44 previously synthesized compounds (thiazole chalcones, flavanones, flavones, 3-hydroxyflavones, and their acetylated derivatives). The concerned compounds have shown antitumoral hallmarks and antibacterial activity in vitro. The experimental method used to determine compounds’ lipophilicity was the reverse-phase thin layer chromatography (RP-TLC). Lipophilicity related 0 parameters—isocratic retention factor (RM), relative lipophily (RM ), slope (b), chromatographic hydrophobic index ('0), scores of principal components (PC1/RM)—were determined based on reverse-phase chromatography results. Keywords: lipophilicity; retention factor; chalcones; QSAR; chromatography; drug design 1. Introduction Lipophilicity is an important feature of molecules in pharmaceutical, biochemical, and medical chemistry fields. -

Solvent Effect Modelling of Isocyanuric Products Synthesis by Chemometric Methods
Journal of Automated Methods & Management in Chemistry Vol. 24, No. 4 (July–August 2002) pp. 111–119 Solvent e´ ect modelling of isocyanuric products synthesis by chemometric methods Jean-Louis Havet, Myriam Billiau-Loreau, electric constant) or by empirical parameters (Dimroth± Catherine Porte* and Alain Delacroix Reichardt constant, Kosower constant). These data can Laboratoire de Chimie Industrielle et Geè nie des Proceè deè s, Conservatoire National be considered as good but partial indicators of polarity. des Arts et Meè tiers, 2 rue Conteè , F-75003 Paris, France Furthermore, the accumulation of empirical values shows that none of these de® nitions is totally convenient. The Chemometric tools were used to generate the modelling of solvent use of several parameters that are not independent in N e¡ects on the -alkylation of an isocyanuric acid salt. The method multiparametric equations allows one to improve the proceeded from a central composite design applied on the Carlson quanti® cation of polarity, but it remains insuæ cient to solvent classi¢cation using principal components analysis. The have a general classi® cation of solvents [3]. selectivity of the reaction was studied from the production of di¡erent substituted isocyanuric derivatives. Response graphs were The most ambitious approach for a general classi® cation obtained for each compound and used to devise a strategy for of solvents uses multivariate statistical methods [4, 5]. solvent selection. The prediction models were validated and used to The compilation of the physicochemical constants of search for the best selectivity for the reaction system. The solvent solvents allows one to take diå erent properties simulta- most often selected as the best for the reaction is the N,N- neously into account. -

Effect of Pressure on Distillation Separation Operation
Solutions for R&D to Design PreFEED Effect of Pressure on Distillation Separation Operation April 30, 2012 PreFEED Corporation Hiromasa Taguchi Solutions for R&D to Design 1 PreFEED Introduction Generally, it is known that, for distillation, separation tends to be enhanced by lower pressures. For two components, the ease of distillation separation can be judged from the value of the relative volatility (α). Here, we will take typical substances as examples and examine the effect of pressure changes on their relative volatilities. Solutions for R&D to Design 2 PreFEED Ideal Solution System The relative volatility (α) is a ratio of vapor-liquid equilibrium ratios and can be expressed by the following equation: K1 y1 y2 K1 , K2 K2 x1 x2 In the case of an ideal solution system , using Raoult’s law, the relative volatility can be expressed as a ratio of vapor pressures. Py x Po 1 1 1 Raoult’s law Py x Po o 2 2 2 K1 P1 y Po o K 1 1 K2 P2 1 x1 P o y2 P2 K2 x2 P Solutions for R&D to Design 3 PreFEED Methanol-Ethanol System As an example of an ideal solution system , let’s consider a methanol-ethanol binary system. α is calculated by obtaining vapor pressures from the Antoine constants in the table below. 0 B 2.8 ln(Pi [Pa]) A 2.6 C T[K] 2.4 ABC 2.2 Methanol 23.4803 3626.55 -34.29 2 Ethanol 23.8047 3803.98 -41.68 1.8 As the temperature (saturated 1.6 pressure) decreases, the value of 1.4 α increases.