Vaccinium Oxycoccos L., Ericaceae) Clones in Lithuanian Reserves
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Checklist of Common Native Plants the Diversity of Acadia National Park Is Refl Ected in Its Plant Life; More Than 1,100 Plant Species Are Found Here
National Park Service Acadia U.S. Department of the Interior Acadia National Park Checklist of Common Native Plants The diversity of Acadia National Park is refl ected in its plant life; more than 1,100 plant species are found here. This checklist groups the park’s most common plants into the communities where they are typically found. The plant’s growth form is indicated by “t” for trees and “s” for shrubs. To identify unfamiliar plants, consult a fi eld guide or visit the Wild Gardens of Acadia at Sieur de Monts Spring, where more than 400 plants are labeled and displayed in their habitats. All plants within Acadia National Park are protected. Please help protect the park’s fragile beauty by leaving plants in the condition that you fi nd them. Deciduous Woods ash, white t Fraxinus americana maple, mountain t Acer spicatum aspen, big-toothed t Populus grandidentata maple, red t Acer rubrum aspen, trembling t Populus tremuloides maple, striped t Acer pensylvanicum aster, large-leaved Aster macrophyllus maple, sugar t Acer saccharum beech, American t Fagus grandifolia mayfl ower, Canada Maianthemum canadense birch, paper t Betula papyrifera oak, red t Quercus rubra birch, yellow t Betula alleghaniesis pine, white t Pinus strobus blueberry, low sweet s Vaccinium angustifolium pyrola, round-leaved Pyrola americana bunchberry Cornus canadensis sarsaparilla, wild Aralia nudicaulis bush-honeysuckle s Diervilla lonicera saxifrage, early Saxifraga virginiensis cherry, pin t Prunus pensylvanica shadbush or serviceberry s,t Amelanchier spp. cherry, choke t Prunus virginiana Solomon’s seal, false Maianthemum racemosum elder, red-berried or s Sambucus racemosa ssp. -

Natural Landscapes of Maine a Guide to Natural Communities and Ecosystems
Natural Landscapes of Maine A Guide to Natural Communities and Ecosystems by Susan Gawler and Andrew Cutko Natural Landscapes of Maine A Guide to Natural Communities and Ecosystems by Susan Gawler and Andrew Cutko Copyright © 2010 by the Maine Natural Areas Program, Maine Department of Conservation 93 State House Station, Augusta, Maine 04333-0093 All rights reserved. No part of this book may be reproduced or transmitted in any form or by any means, electronic or mechanical, including photocopying, recording, or by any information storage and retrieval system without written permission from the authors or the Maine Natural Areas Program, except for inclusion of brief quotations in a review. Illustrations and photographs are used with permission and are copyright by the contributors. Images cannot be reproduced without expressed written consent of the contributor. ISBN 0-615-34739-4 To cite this document: Gawler, S. and A. Cutko. 2010. Natural Landscapes of Maine: A Guide to Natural Communities and Ecosystems. Maine Natural Areas Program, Maine Department of Conservation, Augusta, Maine. Cover photo: Circumneutral Riverside Seep on the St. John River, Maine Printed and bound in Maine using recycled, chlorine-free paper Contents Page Acknowledgements ..................................................................................... 3 Foreword ..................................................................................................... 4 Introduction ............................................................................................... -

Vaccinium Oxycoccos L
Plant Guide these fruits in their food economy (Waterman 1920). SMALL CRANBERRY Small cranberries were gathered wild in England and Vaccinium oxycoccos L. Scotland and made into tarts, marmalade, jelly, jam, Plant Symbol = VAOX and added to puddings and pies (Eastwood 1856). Many colonists were already familiar with this fruit Contributed by: USDA NRCS National Plant Data in Great Britain before finding it in North America. Team, Greensboro, NC The small cranberry helped stock the larder of English and American ships, fed trappers in remote regions, and pleased the palates of Meriwether Lewis and William Clark in their explorations across the United States (Lewis and Clark 1965). The Chinook, for example, traded dried cranberries with the English vessel Ruby in 1795 and at Thanksgiving in 1805 Lewis and Clark dined on venison, ducks, geese, and small cranberry sauce from fruit brought by Chinook women (McDonald 1966; Lewis and Clark 1965). Because the small cranberry can grow in association with large cranberry (Vaccinium macrocarpon) in the Great Lakes region, northeastern USA and southeastern Canada (Boniello 1993; Roger Latham pers. comm. 2009) it is possible that the Pilgrims of Plymouth were introduced to both edible Small cranberries growing in a bog on the western Olympic species by the Wampanoag. Peninsula, Washington. Photograph by Jacilee Wray, 2006. The berries are still gathered today in the United Alternate Names States, Canada, and Europe (Himelrick 2005). The Bog cranberry, swamp cranberry, wild cranberry Makah, Quinault, and Quileute of the Olympic Peninsula still gather them every fall and non-Indians Uses from early settler families still gather them (Anderson Said to have a superior flavor to the cultivated 2009). -

Cranberry - Wikipedia
8/16/2018 Cranberry - Wikipedia Cranberry Cranberries are a group of evergreen dwarf shrubs or trailing vines in the Cranberry subgenus Oxycoccus of the genus Vaccinium. In Britain, cranberry may refer to the native species Vaccinium oxycoccos,[1] while in North America, cranberry may refer to Vaccinium macrocarpon.[2] Vaccinium oxycoccos is cultivated in central and northern Europe, while Vaccinium macrocarpon is cultivated throughout the northern United States, Canada and Chile.[3] In some methods of classification, Oxycoccus is regarded as a genus in its own right.[4] They can be found in acidic bogs throughout the cooler regions of the Northern Hemisphere. Cranberry bush with fruit partially Cranberries are low, creeping shrubs or vines up to 2 meters (7 ft) long and 5 submerged to 20 centimeters (2 to 8 in) in height;[5] they have slender, wiry stems that are not thickly woody and have small evergreen leaves. The flowers are dark Scientific classification pink, with very distinct reflexed petals, leaving the style and stamens fully Kingdom: Plantae exposed and pointing forward. They are pollinated by bees. The fruit is a berry that is larger than the leaves of the plant; it is initially light green, Clade: Angiosperms turning red when ripe. It is edible, but with an acidic taste that usually Clade: Eudicots overwhelms its sweetness. Clade: Asterids Cranberries are a major commercial crop in certain American states and Order: Ericales Canadian provinces (see cultivation and uses below). Most cranberries are processed into products such as juice, sauce, jam, and sweetened dried Family: Ericaceae cranberries, with the remainder sold fresh to consumers. -

Exploring the Genetic Diversity of Wild Cranberry Populations in the Upper Midwestern United States
Published October 24, 2019 RESEARCH Exploring the Genetic Diversity of Wild Cranberry Populations in the Upper Midwestern United States Lorraine Rodríguez-Bonilla,* Fabian Rodríguez Bonilla, Daniel Matusinec, Eric Wiesman, Sean D. Schoville, Amaya Atucha, and Juan Zalapa* L. Rodríguez-Bonilla, A. Atucha, and J. Zalapa, Dep. of Horticulture, ABSTRACT Univ. of Wisconsin, Madison, WI 53706; F.R. Bonilla, Dep. of Biology, Plant breeding continuously evolves to satisfy Univ. of Puerto Rico Mayaguez, Mayaguez, Puerto Rico 00680; D. the needs of the growing population, but in Matusinec, Dep. of Atmospheric and Oceanic Sciences and Political many crops, it has resulted in a decline in the Science, Univ. of Wisconsin, Madison, WI 53706; E. Wiesman and genetic diversity available. Therefore, increasing J. Zalapa, USDA-ARS, Vegetable Crops Research Unit, Univ. of knowledge of the range, genetic relationships, Wisconsin, Madison, WI 53706; S.D. Schoville, Dep. of Entomology, and diversity among crop wild relatives (CWR) Univ. of Wisconsin, Madison, WI 53706. Received 5 June 2019. Accepted is essential for the efficient use of available 30 Sept. 2019. *Corresponding authors ([email protected]; germplasm in breeding programs. In cran- [email protected]). Assigned to Associate Editor Joseph Robins. berries (Vaccinium macrocarpon Ait.), most Abbreviations: CTAB, cetyltrimethylammonium bromide; CWR, cultivars share the same genetic background crop wild relative(s); EDTA, ethylenediaminetetraacetic acid; PCA, based on only a few wild selections. This limits principal component analysis; PCoA, principal coordinate analysis; the breeding pool for selection to support the PC, principal component; PCR, polymerase chain reaction; RAPD, cranberry industry. Therefore, we studied 36 random amplified polymorphic DNA; SSR, simple sequence repeat. -

Anti-Carcinoma Activity of Vaccinium Oxycoccos
Available online at www.scholarsresearchlibrary.com Scholars Research Library Der Pharmacia Lettre, 2017, 9 (3):74-79 (http://scholarsresearchlibrary.com/archive.html) ISSN 0975-5071 USA CODEN: DPLEB4 Anti-carcinoma activity of Vaccinium oxycoccos Mansoureh Masoudi1, Milad Saiedi2* 1Valiasr Eghlid hospital, Shiraz University of medical sciences, Shiraz, Iran 2student of medicine, international Pardis University of Yazd, Yazd, Iran *Corresponding author: Milad Saiedi, student of medicine, international Pardis University of Yazd, Yazd, Iran _______________________________________________________ ABSTRACT Introduction: Vaccinium oxycoccos or cranberry are evergreen shrubs in the subgenus Oxycoccos of the genus Vaccinium. The aim of this study was to overview anti-carcinoma activity of Vaccinium oxycoccos. Methods: This review article was carried out by searching studies in PubMed, Medline, Web of Science, and IranMedex databases. The initial search strategy identified about 79 references. In this study, 56 studies were accepted for further screening and met all our inclusion criteria [in English, full text, therapeutic effects of Vaccinium oxycoccos and dated mainly from the year 2002 to 2016.The search terms were “Vaccinium oxycoccos”, “therapeutic properties”, “pharmacological effects”. Result: the result of this study showed that Vaccinium oxycoccos possess anti-carcinoma activity against the following cancers: prostate, bladder, lymphoma, ovarian, cervix, breast, lung, and colon. Conclusion: the results from this review are quite promising for the use of Vaccinium oxycoccos as an anti-cancer agent. Vaccinium oxycoccos possess the ability to suppress the proliferation of human breast cancer MCF-7 cells and this suppression is at least partly attributed to both the initiation of apoptosis and the G1 phase arrest. Keywords: Vaccinium oxycoccos, Phytochemicals, Therapeutic effects, Pharmacognosy, Alternative and complementary medicine. -

Chapter 5: Vegetation of Sphagnum-Dominated Peatlands
CHAPTER 5: VEGETATION OF SPHAGNUM-DOMINATED PEATLANDS As discussed in the previous chapters, peatland ecosystems have unique chemical, physical, and biological properties that have given rise to equally unique plant communities. As indicated in Chapter 1, extensive literature exists on the classification, description, and ecology of peatland ecosystems in Europe, the northeastern United States, Canada, and the Rocky Mountains. In addition to the references cited in Chapter 1, there is some other relatively recent literature on peatlands (Verhoeven 1992; Heinselman 1963, 1970; Chadde et al., 1998). Except for efforts on the classification and ecology of peatlands in British Columbia by the National Wetlands Working Group (1988), the Burns Bog Ecosystem Review (Hebda et al. 2000), and the preliminary classification of native, low elevation, freshwater vegetation in western Washington (Kunze 1994), scant information exists on peatlands within the more temperate lowland or maritime climates of the Pacific Northwest (Oregon, Washington, and British Columbia). 5.1 Introduction There are a number of classification schemes and many different peatland types, but most use vegetation in addition to hydrology, chemistry and topological characteristics to differentiate among peatlands. The subject of this report are acidic peatlands that support acidophilic (acid-loving) and xerophytic vegetation, such as Sphagnum mosses and ericaceous shrubs. Ecosystems in Washington state appear to represent a mosaic of vegetation communities at various stages of succession and are herein referred to collectively as Sphagnum-dominated peatlands. Although there has been some recognition of the unique ecological and societal values of peatlands in Washington, a statewide classification scheme has not been formally adopted or widely recognized in the scientific community. -

Botanical Name Common Name
Approved Approved & as a eligible to Not eligible to Approved as Frontage fulfill other fulfill other Type of plant a Street Tree Tree standards standards Heritage Tree Tree Heritage Species Botanical Name Common name Native Abelia x grandiflora Glossy Abelia Shrub, Deciduous No No No Yes White Forsytha; Korean Abeliophyllum distichum Shrub, Deciduous No No No Yes Abelialeaf Acanthropanax Fiveleaf Aralia Shrub, Deciduous No No No Yes sieboldianus Acer ginnala Amur Maple Shrub, Deciduous No No No Yes Aesculus parviflora Bottlebrush Buckeye Shrub, Deciduous No No No Yes Aesculus pavia Red Buckeye Shrub, Deciduous No No Yes Yes Alnus incana ssp. rugosa Speckled Alder Shrub, Deciduous Yes No No Yes Alnus serrulata Hazel Alder Shrub, Deciduous Yes No No Yes Amelanchier humilis Low Serviceberry Shrub, Deciduous Yes No No Yes Amelanchier stolonifera Running Serviceberry Shrub, Deciduous Yes No No Yes False Indigo Bush; Amorpha fruticosa Desert False Indigo; Shrub, Deciduous Yes No No No Not eligible Bastard Indigo Aronia arbutifolia Red Chokeberry Shrub, Deciduous Yes No No Yes Aronia melanocarpa Black Chokeberry Shrub, Deciduous Yes No No Yes Aronia prunifolia Purple Chokeberry Shrub, Deciduous Yes No No Yes Groundsel-Bush; Eastern Baccharis halimifolia Shrub, Deciduous No No Yes Yes Baccharis Summer Cypress; Bassia scoparia Shrub, Deciduous No No No Yes Burning-Bush Berberis canadensis American Barberry Shrub, Deciduous Yes No No Yes Common Barberry; Berberis vulgaris Shrub, Deciduous No No No No Not eligible European Barberry Betula pumila -
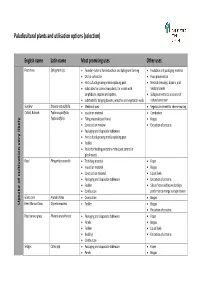
List of Paludicultural Plants and Utilisation Options (Selection)
Paludicultural plants and utilisation options (selection) English name Latin name Most promising uses Other uses Peat moss Sphagnum spp. Founder material for restoration and Sphagnum farming Insulation and packaging material Orchid cultivation Food preservation Horticultural growing media replacing peat Medical dressings, diapers, and substrates for carnivorous plants, for vivaria with sanitary towels amphibians, reptiles and spiders, Sphagnum extracts as source of substrate for hanging baskets, wreathes and vegetation walls natural sunscreen Sundew Drosera rotundifolia Medicinal uses Vegetarian rennet for cheese making Cattail, Bulrush Typha angustifolia, Insulation material Combustion Typha latifolia Filling material (seed hairs) Biogas Construction material Extraction of proteins likely Packaging and disposable tableware Horticultural growing media replacing peat very Fodder Pollen for feeding predatory mites (pest control in glasshouses) Reed Phragmites australis Thatching material Paper Insulation material Biogas Construction material Liquid fuels cultivation Packaging and disposable tableware Extraction of proteins of Fodder Silicon from reed leaves for high‐ Combustion performance energy storage devices Giant cane Arundo donax Combustion Biogas Uptake Reed Manna Grass Glyceria maxima Fodder Biogas Extraction of proteins Reed canary grass Phalaris arundinacea Packaging and disposable tableware Paper Panels Biogas Fodder Liquid fuels Bedding Extraction of proteins Combustion Sedges Carex -

Vegetation Classification and Mapping Project Report
U.S. Geological Survey-National Park Service Vegetation Mapping Program Acadia National Park, Maine Project Report Revised Edition – October 2003 Mention of trade names or commercial products does not constitute endorsement or recommendation for use by the U. S. Department of the Interior, U. S. Geological Survey. USGS-NPS Vegetation Mapping Program Acadia National Park U.S. Geological Survey-National Park Service Vegetation Mapping Program Acadia National Park, Maine Sara Lubinski and Kevin Hop U.S. Geological Survey Upper Midwest Environmental Sciences Center and Susan Gawler Maine Natural Areas Program This report produced by U.S. Department of the Interior U.S. Geological Survey Upper Midwest Environmental Sciences Center 2630 Fanta Reed Road La Crosse, Wisconsin 54603 and Maine Natural Areas Program Department of Conservation 159 Hospital Street 93 State House Station Augusta, Maine 04333-0093 In conjunction with Mike Story (NPS Vegetation Mapping Coordinator) NPS, Natural Resources Information Division, Inventory and Monitoring Program Karl Brown (USGS Vegetation Mapping Coordinator) USGS, Center for Biological Informatics and Revised Edition - October 2003 USGS-NPS Vegetation Mapping Program Acadia National Park Contacts U.S. Department of Interior United States Geological Survey - Biological Resources Division Website: http://www.usgs.gov U.S. Geological Survey Center for Biological Informatics P.O. Box 25046 Building 810, Room 8000, MS-302 Denver Federal Center Denver, Colorado 80225-0046 Website: http://biology.usgs.gov/cbi Karl Brown USGS Program Coordinator - USGS-NPS Vegetation Mapping Program Phone: (303) 202-4240 E-mail: [email protected] Susan Stitt USGS Remote Sensing and Geospatial Technologies Specialist USGS-NPS Vegetation Mapping Program Phone: (303) 202-4234 E-mail: [email protected] Kevin Hop Principal Investigator U.S. -

Table S1. Priority Species of Medicinal Plants Achillea Millefolium L
Table S1. Priority species of medicinal plants Achillea millefolium L. Geranium robertianum L. Pulsatilla pratensis (L.) Mill. Acorus calamus L. Geum rivale L. Quercus robur L. Agrimonia eupatoria L. Geum urbanum L. Rhamnus cathartica L. Alchemilla vulgaris L. Glechoma hederacea L. Ribes nigrum L. Allium angulosum L. Gratiola officinalis L. Rosa canina L. Allium oleraceum L. Helichrysum arenarium (L.) Moench Rubus caesius L. Allium scorodoprasum L. Hepatica nobilis Mill. Rubus chamaemorus L. Allium ursinum L. Herniaria glabra L. Rubus idaeus L. Allium vineale L. Hippophae rhamnoides L. Rubus nessensis Hall Alnus glutinosa (L.) Gaertn. Humulus lupulus L. Rubus plicatus Weihe & Nees Alnus incana (L.) Moench Hypericum maculatum Crantz Rumex acetosa L. Angelica archangelica L. Hypericum perforatum L. Rumex crispus L. Antennaria dioica (L.) Gaertn. Juniperus communis L. Rumex thyrsiflorus Fingerh. Arctostaphylos uva-ursi (L.) Spreng. Lamium album L. Salix ×fragilis L. Arnica montana L. Ledum palustre L. Salix purpurea L. Artemisia absinthium L. Leonurus cardiaca L. Sanguisorba officinalis L. Artemisia vulgaris L. Linaria vulgaris Mill. Saponaria officinalis L. Berberis vulgaris L. Lithospermum officinale L. Scrophularia nodosa L. Betula pendula Roth Lycopodium clavatum L. Sedum acre L. Betula pubescens Ehrh. Lycopus europaeus L. Solanum dulcamara L. Calluna vulgaris (L.) Hull Lythrum salicaria L. Solidago virgaurea L. Carex arenaria L. Mentha aquatica L. Sorbus aucuparia L. Carum carvi L. Mentha arvensis L. Stachys officinalis (L.) Trevis. Centaurium erythraea Rafn Menyanthes trifoliata L. Symphytum officinale L. Chelidonium majus L. Myrica gale L. Tanacetum vulgare L. Cichorium intybus L. Oenothera biennis L. Taraxacum campylodes G.E.Haglund Comarum palustre L. Origanum vulgare L. Thymus pulegioides L. -

Plant Fungal Interactions & Vegetation Change Under Elevated Nitrogen Supply
EnhancedEnhanced nitrogennitrogen supplysupply leadsleads toto changedchanged plantplant chemistrychemistry andand vegetationvegetation shiftshift inin borealboreal miremire Magdalena Wiedermann*, Annika Nordin, Mats Nilsson, Urban Gunnarsson & Lars Ericson *Department of Ecology and Environmental Science Umeå University TotalTotal NN depositiondeposition inin (kg(kg haha-1 yryr-1)) SwedenSweden Degerö Stormyr 1 – 1.5 1.5 – 2 2 – 2.5 2.5 – 3 3 – 4 4 – 5 5 – 6 6 – 7 7 – 9 9 – 12 12 – 15 15 – 18 Swedish Meteorological and Hydrological Institute (SMHI; 26 November 2001) DegerDegeröö StormyrStormyr KulbKulbääckslidencksliden full factorial design with N, S and T treatment treatments were applied since 1995: • ammonium nitrate (30kg N ha-1 yr-1) • sodium sulphate (20kg S ha-1 yr-1) • greenhouses enhance air temp 3.6°C above ..ambient fieldwork Study species (clockwise from upper left): Andromeda polifolia, Eriophorum vaginatum, Sphagnum balticum, S. lindbergii, S.majus, Vaccinium oxycoccos, S. papillosum treatment responses control sulfur high nitrogen nitrogen & temperature Vegetation cover of the three vascular plants in % 45 Eriophorum vaginatum Andromeda polifolia 40 Vaccinium oxycoccos 35 30 25 20 % cover 15 10 5 0 c S t St ns N NS Nt NSt factors d.f. F model sig. R2 Sig. model factors E. vaginatum F1,15 48.7 *** 0.76 +T*** A. polifolia F2,14 14.6 *** 0.68 +N*** +T** V. oxycoccos F1,15 4.6 * 0.24 +N* Leave nitrogen content of the three vascular plants in % dry weight Eriophorum vaginatum Andromeda polifolia Vaccinium oxycoccos 1.6 1.5 1.4 1.3 1.2 % dry mass 1.1 1.0 0.9 0.8 c S t St ns N NS Nt NSt factors d.f F model sig.