Redalyc.Gastropod Communities Associated with Ulva Spp. in The
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Introduced Aquatic Species in California Open Coastal Waters -2007
INTRODUCED AQUATIC SPECIES IN CALIFORNIA OPEN COASTAL WATERS -2007 Final Report September 2008 California Department of Fish and Game Office of Spill Prevention and Response Moss Landing Marine Laboratories Marine Pollution Studies Laboratory 1 AUTHORS Erin R. Maloney, Russell Fairey, Ashleigh Lyman, Zea Walton, Marco Sigala Moss Landing Marine Laboratories/Marine Pollution Studies Laboratory This report should be cited as: Maloney, E., Fairey, R., Lyman, A., Walton, Z., Sigala, M. 2007. Introduced Aquatic Species in California’s Open Coastal Waters - 2007. Final Report. California Department of Fish and Game, Sacramento, CA., 84 pp. 2 ACKNOWLEDGEMENTS This study was completed thanks to the efforts of the following individuals: California Department of Fish and Game: Project Management and Reporting Steve Foss, Michael Sowby, Peter Ode San Jose State University Foundation- Moss Landing Marine Laboratories Marine Pollution Studies Laboratory: Project oversight, field sampling, literature review and data analysis: Russell Fairey, Erin Maloney, Ashleigh Lyman, Mark Pranger, Marco Sigala, Cassandra Lamerdin, Zea Walton, Paul Tompkins, Kim Quaranta, Aurora Alifano, Selena McMillan, Jon Walsh, Jason Felton, Lewis Barnett, Vince Christian. Marine Benthic Ecology Laboratory: Project oversight, training, sample transfer, sorting and gross taxonomy, data entry: Aurora Alifano, Stepheni Ceperley, Cori Gibble, Kamille Hammerstrom, Andy Hansen, Scott Hansen, Brian Hoover, Erin Jensen, Jim Oakden, John Oliver, Sierra Perry, Jasmine Ruvalcaba, Jared -

ABSTRACT Title of Dissertation: PATTERNS IN
ABSTRACT Title of Dissertation: PATTERNS IN DIVERSITY AND DISTRIBUTION OF BENTHIC MOLLUSCS ALONG A DEPTH GRADIENT IN THE BAHAMAS Michael Joseph Dowgiallo, Doctor of Philosophy, 2004 Dissertation directed by: Professor Marjorie L. Reaka-Kudla Department of Biology, UMCP Species richness and abundance of benthic bivalve and gastropod molluscs was determined over a depth gradient of 5 - 244 m at Lee Stocking Island, Bahamas by deploying replicate benthic collectors at five sites at 5 m, 14 m, 46 m, 153 m, and 244 m for six months beginning in December 1993. A total of 773 individual molluscs comprising at least 72 taxa were retrieved from the collectors. Analysis of the molluscan fauna that colonized the collectors showed overwhelmingly higher abundance and diversity at the 5 m, 14 m, and 46 m sites as compared to the deeper sites at 153 m and 244 m. Irradiance, temperature, and habitat heterogeneity all declined with depth, coincident with declines in the abundance and diversity of the molluscs. Herbivorous modes of feeding predominated (52%) and carnivorous modes of feeding were common (44%) over the range of depths studied at Lee Stocking Island, but mode of feeding did not change significantly over depth. One bivalve and one gastropod species showed a significant decline in body size with increasing depth. Analysis of data for 960 species of gastropod molluscs from the Western Atlantic Gastropod Database of the Academy of Natural Sciences (ANS) that have ranges including the Bahamas showed a positive correlation between body size of species of gastropods and their geographic ranges. There was also a positive correlation between depth range and the size of the geographic range. -

Portadas 22 (1)
© Sociedad Española de Malacología Iberus , 22 (1): 43-75, 2004 Gastropods collected along the continental slope of the Colombian Caribbean during the INVEMAR-Macrofauna campaigns (1998-2001) Gasterópodos colectados en el talud continental del Caribe colom - biano durante las campañas INVEMAR-Macrofauna (1998-2001) Adriana GRACIA C. , Néstor E. ARDILA and Juan Manuel DÍAZ* Recibido el 26-III-2003. Aceptado el 5-VII-2003 ABSTRACT Among the biological material collected during the 1998-2001 “INVEMAR-Macrofauna” campaigns aboard the R/V Ancón along the upper zone of the continental slope of the Colombian Caribbean, at depths ranging from 200 to 520 m, a total of 104 gastropod species were obtained. Besides 18 not yet identified species, but including one recently described new species ( Armina juliana Ardila and Díaz, 2002), 48 species were not pre - viously known from Colombia, 18 of which were also unknown from the Caribbean Sea. Of the 36 families represented, Turridae was by far the richest in species (26 species). An annotated list of the taxa recorded is provided, as well as illustrations of those recorded for the first time in the area. RESUMEN Entre el material biológico colectado en 1998-2001 durante las campañas “INVEMAR- Macrofauna” a bordo del B/I Ancón , a profundidades entre 200 y 520 m, se obtuvo un total de 104 especies de gasterópodos. Aparte de 18 especies cuya identificación no ha sido completada, pero incluyendo una especie recientemente descrita ( Armina juliana Ardila y Díaz, 2002), 48 especies no habían sido registradas antes en aguas colombia - nas y 18 de ellas tampoco en el mar Caribe. -

2008 Trough to Trough
Trough to trough The Colorado River and the Salton Sea Robert E. Reynolds, editor The Salton Sea, 1906 Trough to trough—the field trip guide Robert E. Reynolds, George T. Jefferson, and David K. Lynch Proceedings of the 2008 Desert Symposium Robert E. Reynolds, compiler California State University, Desert Studies Consortium and LSA Associates, Inc. April 2008 Front cover: Cibola Wash. R.E. Reynolds photograph. Back cover: the Bouse Guys on the hunt for ancient lakes. From left: Keith Howard, USGS emeritus; Robert Reynolds, LSA Associates; Phil Pearthree, Arizona Geological Survey; and Daniel Malmon, USGS. Photo courtesy Keith Howard. 2 2008 Desert Symposium Table of Contents Trough to trough: the 2009 Desert Symposium Field Trip ....................................................................................5 Robert E. Reynolds The vegetation of the Mojave and Colorado deserts .....................................................................................................................31 Leah Gardner Southern California vanadate occurrences and vanadium minerals .....................................................................................39 Paul M. Adams The Iron Hat (Ironclad) ore deposits, Marble Mountains, San Bernardino County, California ..................................44 Bruce W. Bridenbecker Possible Bouse Formation in the Bristol Lake basin, California ................................................................................................48 Robert E. Reynolds, David M. Miller, and Jordon Bright Review -

Florida Keys Species List
FKNMS Species List A B C D E F G H I J K L M N O P Q R S T 1 Marine and Terrestrial Species of the Florida Keys 2 Phylum Subphylum Class Subclass Order Suborder Infraorder Superfamily Family Scientific Name Common Name Notes 3 1 Porifera (Sponges) Demospongia Dictyoceratida Spongiidae Euryspongia rosea species from G.P. Schmahl, BNP survey 4 2 Fasciospongia cerebriformis species from G.P. Schmahl, BNP survey 5 3 Hippospongia gossypina Velvet sponge 6 4 Hippospongia lachne Sheepswool sponge 7 5 Oligoceras violacea Tortugas survey, Wheaton list 8 6 Spongia barbara Yellow sponge 9 7 Spongia graminea Glove sponge 10 8 Spongia obscura Grass sponge 11 9 Spongia sterea Wire sponge 12 10 Irciniidae Ircinia campana Vase sponge 13 11 Ircinia felix Stinker sponge 14 12 Ircinia cf. Ramosa species from G.P. Schmahl, BNP survey 15 13 Ircinia strobilina Black-ball sponge 16 14 Smenospongia aurea species from G.P. Schmahl, BNP survey, Tortugas survey, Wheaton list 17 15 Thorecta horridus recorded from Keys by Wiedenmayer 18 16 Dendroceratida Dysideidae Dysidea etheria species from G.P. Schmahl, BNP survey; Tortugas survey, Wheaton list 19 17 Dysidea fragilis species from G.P. Schmahl, BNP survey; Tortugas survey, Wheaton list 20 18 Dysidea janiae species from G.P. Schmahl, BNP survey; Tortugas survey, Wheaton list 21 19 Dysidea variabilis species from G.P. Schmahl, BNP survey 22 20 Verongida Druinellidae Pseudoceratina crassa Branching tube sponge 23 21 Aplysinidae Aplysina archeri species from G.P. Schmahl, BNP survey 24 22 Aplysina cauliformis Row pore rope sponge 25 23 Aplysina fistularis Yellow tube sponge 26 24 Aplysina lacunosa 27 25 Verongula rigida Pitted sponge 28 26 Darwinellidae Aplysilla sulfurea species from G.P. -

University of Cincinnati
U UNIVERSITY OF CINCINNATI Date: I, , hereby submit this original work as part of the requirements for the degree of: in It is entitled: Student Signature: This work and its defense approved by: Committee Chair: Approval of the electronic document: I have reviewed the Thesis/Dissertation in its final electronic format and certify that it is an accurate copy of the document reviewed and approved by the committee. Committee Chair signature: Environmental Change and Molluscan Death Assemblages: An Assessment of Ecological History Along a Carbonate Bank in Florida Bay A dissertation submitted to the Graduate School of the University of Cincinnati In partial fulfillment of the requirements for the degree of DOCTOR OF PHILOSOPHY (Ph.D.) In the Department of Geology Of the McMicken College of Arts and Sciences 2009 by Chad Allen Ferguson B.S. Hope College, 2000 M.S. University of Cincinnati, 2003 Advisor: Dr. Arnold I. Miller Committee Members: Dr. Carlton E. Brett Dr. David L. Meyer Dr. Linda C. Ivany Dr. Richard B. Aronson Abstract Marine death assemblages typically reflect the ecological and environmental signatures of the settings in which they are deposited. Comparatively little is known, however, about the effects of environmental change on death assemblages or with what acuity past changes might be preserved, by proxy, in sedimentary deposits. This study assesses the response of present-day molluscan death assemblages, both among surface sediments and stratigraphically, to an instance of anthropogenic environmental change along a carbonate bank. Additionally, I investigate whether body size changes can be recognized among death assemblages as a component of a broader response to changing environmental conditions. -
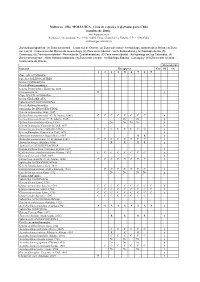
Moluscos - Filo MOLLUSCA
Moluscos - Filo MOLLUSCA. Lista de especies registradas para Cuba (octubre de 2006). José Espinosa Sáez Instituto de Oceanología, Ave 1ª No. 18406, Playa, Ciudad de La Habana, C.P. 11200, Cuba [email protected] Zonas biogeográficas: (1) Zona suroriental – Costa sur de Oriente, (2) Zona surcentral - Archipiélago Jardines de la Reina, (3) Zona sur central - Costa al sur del Macizo de Guamuhaya, (4) Zona suroccidental - Golfo de Batabanó y Archipiélago de los, (5) Canarreos, (6) Zona suroccidental - Península de Guanahacabibes, (7) Zona noroccidental - Archipiélago de Los Colorados, (8) Zona noroccidental - Norte Habana-Matanzas, (9) Zona norte-central - Archipiélago Sabana - Camagüey, (10) Zona norte-oriental - Costa norte de Oriente Abreviaturas Especies Bioegiones Cu Pl Oc 1 2 3 4 5 6 7 8 9 Clase APLACOPHORA Subclase SOLENOGASTRES Orden CAVIBELONIA Familia Proneomeniidae Género Proneomenia Hubrecht, 1880 Proneomenia sp . R x Clase POLYPLACOPHORA Orden NEOLORICATA Suborden ISCHNOCHITONINA Familia Ischnochitonidae Subfamilia ISCHNOCHITONINAE Género Ischnochiton Gray, 1847 Ischnochiton erythronotus (C. B. Adams, 1845) C C C C C C C C x Ischnochiton papillosus (C. B. Adams, 1845) Nc Nc x Ischnochiton striolatus (Gray, 1828) Nc Nc Nc Nc x Género Ischnoplax Carpenter in Dall, 1879 x Ischnoplax pectinatus (Sowerby, 1832) C C C C C C C C x Género Stenoplax Carpenter in Dall, 1879 x Stenoplax bahamensis Kaas y Belle, 1987 R R x Stenoplax purpurascens (C. B. Adams, 1845) C C C C C C C C x Stenoplax boogii (Haddon, 1886) R R R R x Subfamilia CALLISTOPLACINAE Género Callistochiton Carpenter in Dall, 1879 x Callistochiton shuttleworthianus Pilsbry, 1893 C C C C C C C C x Género Ceratozona Dall, 1882 x Ceratozona squalida (C. -

Gastropod Molluscs of the Southern Area Of
PAIDEIA XXI Vol. 10, Nº 2, Lima, julio-diciembre 2020, pp. 289-310 ISSN Versión Impresa: 2221-7770; ISSN Versión Electrónica: 2519-5700 http://revistas.urp.edu.pe/index.php/Paideia ORIGINAL ARTICLE / ARTÍCULO ORIGINAL GASTROPOD MOLLUSCS OF THE SOUTHERN AREA OF CIENFUEGOS, FROM THE BEACH RANCHO LUNA TO THE MOUTH OF THE ARIMAO RIVER, CUBA MOLUSCOS GASTRÓPODOS DE LA ZONA SUR DE CIENFUEGOS, DESDE PLAYA RANCHO LUNA HASTA LA DESEMBOCADURA DEL RÍO ARIMAO, CUBA Oneida Calzadilla-Milian1*; Rafael Armiñana-García2,*; José Alexis Sarría- Martínez1; Rigoberto Fimia-Duarte3; Jose Iannacone4,5; Raiden Grandía- Guzmán6 & Yolepsy Castillo-Fleites2 1* Universidad de Cienfuegos «Carlos Rafael Rodríguez», Cienfuegos, Cuba. E-mail: ocalzadilla@ ucf.edu.cu; [email protected] 2 Universidad Central «Marta Abreu» de Las Villas, Villa Clara, Cuba. E-mail: rarminana@uclv. cu / ycfl [email protected] 3 Facultad de Tecnología de la Salud y Enfermería (FTSE). Universidad de Ciencias Médicas de Villa Clara (UCM-VC), Cuba. E-mail: rigoberto.fi [email protected] 4 Laboratorio de Ecología y Biodiversidad Animal (LEBA). Facultad de Ciencias Naturales y Matemáticas (FCNNM). Universidad Nacional Federico Villarreal (UNFV). Lima, Perú. 5 Facultad de Ciencias Biológicas. Universidad Ricardo Palma (URP). Lima, Perú. E-mail: [email protected] 6 Centro Nacional para la Producción de Animales de Laboratorio (CENPALAB), La Habana, Cuba. E-mail: [email protected] * Author for correspondence: [email protected] ABSTRACT The research presented shows a malacological survey of Cienfuegos' southern area, from “Rancho Luna” beach to the mouth of the “Arimao River”. The malacological studies ranged from January 2018 to December of the same year. -

44-Sep-2016.Pdf
Page 2 Vol. 44, No. 3 In 1972, a group of shell collectors saw the need for a national organization devoted to the interests of shell collec- tors; to the beauty of shells, to their scientific aspects, and to the collecting and preservation of mollusks. This was the start of COA. Our member- AMERICAN CONCHOLOGIST, the official publication of the Conchol- ship includes novices, advanced collectors, scientists, and shell dealers ogists of America, Inc., and issued as part of membership dues, is published from around the world. In 1995, COA adopted a conservation resolution: quarterly in March, June, September, and December, printed by JOHNSON Whereas there are an estimated 100,000 species of living mollusks, many PRESS OF AMERICA, INC. (JPA), 800 N. Court St., P.O. Box 592, Pontiac, IL 61764. All correspondence should go to the Editor. ISSN 1072-2440. of great economic, ecological, and cultural importance to humans and Articles in AMERICAN CONCHOLOGIST may be reproduced with whereas habitat destruction and commercial fisheries have had serious ef- proper credit. We solicit comments, letters, and articles of interest to shell fects on mollusk populations worldwide, and whereas modern conchology collectors, subject to editing. Opinions expressed in “signed” articles are continues the tradition of amateur naturalists exploring and documenting those of the authors, and are not necessarily the opinions of Conchologists the natural world, be it resolved that the Conchologists of America endors- of America. All correspondence pertaining to articles published herein es responsible scientific collecting as a means of monitoring the status of or generated by reproduction of said articles should be directed to the Edi- mollusk species and populations and promoting informed decision making tor. -

A Comparison of Intertidal Species Richness and Composition Between Central California and Oahu, Hawaii
Marine Ecology. ISSN 0173-9565 ORIGINAL ARTICLE A comparison of intertidal species richness and composition between Central California and Oahu, Hawaii Chela J. Zabin1,2, Eric M. Danner3, Erin P. Baumgartner4, David Spafford5, Kathy Ann Miller6 & John S. Pearse7 1 Smithsonian Environmental Research Center, Tiburon, CA, USA 2 Department of Environmental Science and Policy, University of California, Davis, CA, USA 3 Southwest Fisheries Science Center, Santa Cruz, CA, USA 4 Department of Biology, Western Oregon University, Monmouth, OR, USA 5 Department of Botany, University of Hawaii, Manoa, HI, USA 6 University Herbarium, University of California, Berkeley, CA, USA 7 Department of Ecology and Evolutionary Biology, University of California, Santa Cruz, CA, USA Keywords Abstract Climate change; range shifts; rocky shores; temporal comparisons; tropical islands; The intertidal zone of tropical islands is particularly poorly known. In contrast, tropical versus temperate. temperate locations such as California’s Monterey Bay are fairly well studied. However, even in these locations, studies have tended to focus on a few species Correspondence or locations. Here we present the results of the first broadscale surveys of Chela J. Zabin, Smithsonian Environmental invertebrate, fish and algal species richness from a tropical island, Oahu, Research Center, 3152 Paradise Drive, Hawaii, and a temperate mainland coast, Central California. Data were gath- Tiburon, CA 94920, USA. ered through surveys of 10 sites in the early 1970s and again in the mid-1990s E-mail: [email protected] in San Mateo and Santa Cruz counties, California, and of nine sites in 2001– Accepted: 18 August 2012 2005 on Oahu. Surveys were conducted in a similar manner allowing for a comparison between Oahu and Central California and, for California, a com- doi: 10.1111/maec.12007 parison between time periods 24 years apart. -

James Hamilton Mclean: the Master of the Gastropoda
Zoosymposia 13: 014–043 (2019) ISSN 1178-9905 (print edition) http://www.mapress.com/j/zs/ ZOOSYMPOSIA Copyright © 2019 · Magnolia Press ISSN 1178-9913 (online edition) http://dx.doi.org/10.11646/zoosymposia.13.1.4 http://zoobank.org/urn:lsid:zoobank.org:pub:20E93C08-5C32-42FC-9580-1DED748FCB5F James Hamilton McLean: The master of the Gastropoda LINDSEY T. GROVES1, DANIEL L. GEIGER2, JANN E. VENDETTI1, & EUGENE V. COAN3 1Natural History Museum of Los Angeles County, Malacology Department, 900 Exposition Blvd., Los Angeles, California 90007, U.S.A. E-mail: [email protected]; [email protected] 2Santa Barbara Museum of Natural History, Department of Invertebrate Zoology, 2559 Puesta del Sol, Santa Barbara, California 93105, U.S.A. E-mail: [email protected] 3P.O. Box 420495, Summerland Key, Florida 33042, U.S.A. E-mail: [email protected] Abstract A biography of the late James H. McLean, former Curator of Malacology at the Natural History Museum of Los Angeles County is provided. It is complemented with a full bibliography and list of 344 taxa named by him and co-authors (with type information and current status), as well as 40 patronyms. Biography James Hamilton McLean was born in Detroit, Michigan, on June 17, 1936. The McLean family moved to Dobbs Ferry, New York, on the Hudson River in 1940, a short train ride and subway ride away from the American Museum of Natural History (AMNH). His brother Hugh recalled that, “AMNH became the place of choice to go to whenever we could get someone to take us. Those visits opened our eyes to the variety and possibilities of what was out there, waiting for us to discover and collect.” From an early age James seemed destined to have a career at a museum (Figs 1–2). -

Diversity and Community Composition of Marine Mollusks Fauna on a Mainland Island of the Coast of Paraná, Southern Brazil
Pesquisa e Ensino em Ciências ISSN 2526-8236 (online edition) Exatas e da Natureza Pesquisa e Ensino em Ciências 2(1): 48–59 (2018) ARTICLE Research and Teaching in Exatas e da Natureza © 2018 UFCG / CFP / UACEN Exact and Natural Sciences Diversity and community composition of marine mollusks fauna on a mainland island of the coast of Paraná, southern Brazil Marcos de Vasconcellos Gernet1, Eduardo Colley2, Elizângela da Veiga Santos1,3 & Carlos João Birckolz1 (1) Universidade Federal do Paraná, Laboratório de Ecologia Aplicada e Bioinvasões, Rua Rio Grande do Norte 145, Mirassol 83255-000, Pontal do Paraná, Paraná, Brazil. E-mail: [email protected], [email protected] (2) Universidade de São Paulo, Museu de Zoologia, Avenida Nazaré 481, Ipiranga 04263-000, São Paulo, São Paulo, Brazil. E-mail: [email protected] (3) Universidade Federal do Paraná, Setor Litoral, Rua Jaguariaíva, 512, Caiobá 83260-000, Matinhos, Paraná, Brazil. E-mail: [email protected] Gernet M.V., Colley E., Santos E.V. & Birckolz C.J. (2018) Diversity and community composition of marine mollusks fauna on a mainland island of the coast of Paraná, southern Brazil. Pesquisa e Ensino em Ciências Exatas e da Natureza, 2(1): 48–59. http://dx.doi.org/10.29215/pecen.v2i1.580 Diversidade e composição da comunidade de moluscos marinhos de uma ilha continental do litoral do Paraná, sul do Brasil Resumo: A Ilha do Farol é uma ilha continental, no estado do Paraná, sul do Brasil. Devido à sua posição em relação ao continente, três ambientes distintos são observados nela: área de costão rochoso exposto ao mar aberto (A); área estuarina (B); área de praia arenosa (C).