Alkanes Organic Compounds
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

1 5. Chemical Bonding
5. Chemical Bonding: The Covalent Bond Model 5.1 The Covalent Bond Model Almost all chemical substances are found as aggregates of atoms in the form of molecules and ions produced through the reactions of various atoms of elements except the noble-gas elements which are stable mono-atomic gases. Chemical bond is a term that describes the attractive force that is holding the atoms of the same or different kind of atoms in forming a molecule or ionic solid that has more stability than the individual atoms. Depending on the kinds of atoms participating in the interaction there seem to be three types of bonding: Gaining or Losing Electrons: Ionic bonding: Formed between many ions formed by metal and nonmetallic elements. Sharing Electrons: Covalent bonding: sharing of electrons between two atoms of non-metals. Metallic Bonding: sharing of electrons between many atoms of metals. Ionic Compounds Covalent Compounds Metallic Compounds 1. Metal and non-meal Non-metal and non-meal Metal of one type or, element combinations. elements combinations. combinations of two or metal elements combinations. 2. High melting brittle Gases, liquids, or waxy, low Conducting, high melting, crystalline solids. melting soft solids. malleable, ductile crystalline solids. 3. Do not conduct as a solid Do not conduct electricity at Conduct electricity at solid but conducts electricity any state. and molten states. when molten. 4. Dissolved in water produce Most are soluble in non-polar Insoluble in any type of conducting solutions solvents and few in water. solvents. (electrolytes) and few These solutions are non- are soluble in non-polar conducting (non- solvents. -

Chemical Forces Understanding the Relative Melting/Boiling Points of Two
Chapter 8 – Chemical Forces Understanding the relative melting/boiling points of two substances requires an understanding of the forces acting between molecules of those substances. These intermolecular forces are important for many additional reasons. For example, solubility and vapor pressure are governed by intermolecular forces. The same factors that give rise to intermolecular forces (e.g. bond polarity) can also have a profound impact on chemical reactivity. Chemical Forces Internuclear Distances and Atomic Radii There are four general methods of discussing interatomic distances: van der Waal’s, ionic, covalent, and metallic radii. We will discuss the first three in this section. Each has a unique perspective of the nature of the interaction between interacting atoms/ions. Van der Waal's Radii - half the distance between two nuclei of the same element in the solid state not chemically bonded together (e.g. solid noble gases). In general, the distance of separation between adjacent atoms (not bound together) in the solid state should be the sum of their van der Waal’s radii. F F van der Waal's radii F F Ionic Radii – Ionic radii were discussed in Chapter 4 and you should go back and review that now. One further thing is worth mentioning here. Evidence that bonding really exists and is attractive can be seen in ionic radii. For all simple ionic compounds, the ions attain noble gas configurations (e.g. in NaCl the Na+ ion is isoelectronic to neon and the Cl- ion is isoelectronic to argon). For the sodium chloride example just given, van der Waal’s radii would predict (Table 8.1, p. -

Chapter Eleven: Chemical Bonding
© Adrian Dingle’s Chemistry Pages 2004, 2005, 2006, 2007, 2008, 2009, 2010, 2011, 2012. All rights reserved. These materials may NOT be copied or redistributed in any way, except for individual class instruction. Revised August 2011 AP TOPIC 8: Chemical Bonding Introduction In the study of bonding we will consider several different types of chemical bond and some of the theories associated with them. TYPES OF BONDING INTRA INTER (Within (inside) compounds) (Interactions between the STRONG molecules of a compound) WEAK Ionic Covalent Hydrogen Bonding Dipole-Dipole London Dispersion (Metal + Non-metal) (Non-metals) (H attached to N, O or F) (Polar molecules) Forces Giant lattice of ions Discrete molecules Strong, permanent dipole Permanent dipoles (Non-polar molecules) Induced dipoles Dative or (Co-ordinate) (Electron deficient species) Discrete molecules To help distinguish the difference in strength of intra and inter bonds consider the process of boiling of water. When water boils the product is steam (gaseous water). The products are not hydrogen and oxygen. This is because the weak inter molecular forces are broken not the much stronger intra molecular forces. C:\Documents and Settings\AdrianD\My Documents\Dropbox\ADCP\apnotes08.doc Page 1 of 26 © Adrian Dingle’s Chemistry Pages 2004, 2005, 2006, 2007, 2008, 2009, 2010, 2011, 2012. All rights reserved. These materials may NOT be copied or redistributed in any way, except for individual class instruction. Revised August 2011 Intra Bonding Ionic (the transfer of electrons between atoms to form ions that form giant ionic lattices) Atoms have equal numbers of protons and electrons and consequently have no overall charge. -

Polyphenolic Compounds Extracted and Purified from Buddleja Globosa
molecules Article Polyphenolic Compounds Extracted and Purified from Buddleja Globosa Hope (Buddlejaceae) Leaves Using Natural Deep Eutectic Solvents and Centrifugal Partition Chromatography Jeniffer Torres-Vega 1 , Sergio Gómez-Alonso 2 , José Pérez-Navarro 2 , Julio Alarcón-Enos 3 and Edgar Pastene-Navarrete 1,3,* 1 Laboratorio de Farmacognosia, Departamento de Farmacia, Facultad de Farmacia, Universidad de Concepción, Concepción PC4030000, Chile; [email protected] 2 Regional Institute for Applied Scientific Research, Faculty of Chemical Sciences, University of Castilla-La Mancha, PC13071 Castilla-La Mancha, Spain; [email protected] (S.G.-A.); [email protected] (J.P.-N.) 3 Laboratorio de Síntesis y Biotransformación de Productos Naturales, Universidad del Bío-Bío, Chillán PC3800708, Chile; [email protected] * Correspondence: [email protected]; Tel.: +56-(42)-246-3000 Abstract: Chemical profiling of Buddleja globosa was performed by high-performance liquid chro- matography coupled to electrospray ionization (HPLC-DAD-ESI-IT/MS) and quadrupole time-of-flight high-resolution mass spectrometry (HPLC-ESI-QTOF/MS). The identification of 17 main phenolic com- pounds in B. globosa leaf extracts was achieved. Along with caffeoyl glucoside isomers, caffeoylshikimic Citation: Torres-Vega, J.; acid and several verbascoside derivatives (β-hydroxyverbascoside and β-hydroxyisoverbascoside) were Gómez-Alonso, S.; Pérez-Navarro, J.; Alarcón-Enos, J.; Pastene-Navarrete, identified. Among flavonoid compounds, the presence of 6-hydroxyluteolin-7-O-glucoside, quercetin-3- E. Polyphenolic Compounds O-glucoside, luteolin 7-O-glucoside, apigenin 7-O-glucoside was confirmed. Campneoside I, forsytho- Extracted and Purified from Buddleja side B, lipedoside A and forsythoside A were identified along with verbascoside, isoverbascoside, Globosa Hope (Buddlejaceae) Leaves eukovoside and martynoside. -

Basic Concepts of Chemical Bonding
Basic Concepts of Chemical Bonding Cover 8.1 to 8.7 EXCEPT 1. Omit Energetics of Ionic Bond Formation Omit Born-Haber Cycle 2. Omit Dipole Moments ELEMENTS & COMPOUNDS • Why do elements react to form compounds ? • What are the forces that hold atoms together in molecules ? and ions in ionic compounds ? Electron configuration predict reactivity Element Electron configurations Mg (12e) 1S 2 2S 2 2P 6 3S 2 Reactive Mg 2+ (10e) [Ne] Stable Cl(17e) 1S 2 2S 2 2P 6 3S 2 3P 5 Reactive Cl - (18e) [Ar] Stable CHEMICAL BONDSBONDS attractive force holding atoms together Single Bond : involves an electron pair e.g. H 2 Double Bond : involves two electron pairs e.g. O 2 Triple Bond : involves three electron pairs e.g. N 2 TYPES OF CHEMICAL BONDSBONDS Ionic Polar Covalent Two Extremes Covalent The Two Extremes IONIC BOND results from the transfer of electrons from a metal to a nonmetal. COVALENT BOND results from the sharing of electrons between the atoms. Usually found between nonmetals. The POLAR COVALENT bond is In-between • the IONIC BOND [ transfer of electrons ] and • the COVALENT BOND [ shared electrons] The pair of electrons in a polar covalent bond are not shared equally . DISCRIPTION OF ELECTRONS 1. How Many Electrons ? 2. Electron Configuration 3. Orbital Diagram 4. Quantum Numbers 5. LEWISLEWIS SYMBOLSSYMBOLS LEWISLEWIS SYMBOLSSYMBOLS 1. Electrons are represented as DOTS 2. Only VALENCE electrons are used Atomic Hydrogen is H • Atomic Lithium is Li • Atomic Sodium is Na • All of Group 1 has only one dot The Octet Rule Atoms gain, lose, or share electrons until they are surrounded by 8 valence electrons (s2 p6 ) All noble gases [EXCEPT HE] have s2 p6 configuration. -

Organometrallic Chemistry
CHE 425: ORGANOMETALLIC CHEMISTRY SOURCE: OPEN ACCESS FROM INTERNET; Striver and Atkins Inorganic Chemistry Lecturer: Prof. O. G. Adeyemi ORGANOMETALLIC CHEMISTRY Definitions: Organometallic compounds are compounds that possess one or more metal-carbon bond. The bond must be “ionic or covalent, localized or delocalized between one or more carbon atoms of an organic group or molecule and a transition, lanthanide, actinide, or main group metal atom.” Organometallic chemistry is often described as a bridge between organic and inorganic chemistry. Organometallic compounds are very important in the chemical industry, as a number of them are used as industrial catalysts and as a route to synthesizing drugs that would not have been possible using purely organic synthetic routes. Coordinative unsaturation is a term used to describe a complex that has one or more open coordination sites where another ligand can be accommodated. Coordinative unsaturation is a very important concept in organotrasition metal chemistry. Hapticity of a ligand is the number of atoms that are directly bonded to the metal centre. Hapticity is denoted with a Greek letter η (eta) and the number of bonds a ligand has with a metal centre is indicated as a superscript, thus η1, η2, η3, ηn for hapticity 1, 2, 3, and n respectively. Bridging ligands are normally preceded by μ, with a subscript to indicate the number of metal centres it bridges, e.g. μ2–CO for a CO that bridges two metal centres. Ambidentate ligands are polydentate ligands that can coordinate to the metal centre through one or more atoms. – – – For example CN can coordinate via C or N; SCN via S or N; NO2 via N or N. -

Bond Distances and Bond Orders in Binuclear Metal Complexes of the First Row Transition Metals Titanium Through Zinc
Metal-Metal (MM) Bond Distances and Bond Orders in Binuclear Metal Complexes of the First Row Transition Metals Titanium Through Zinc Richard H. Duncan Lyngdoh*,a, Henry F. Schaefer III*,b and R. Bruce King*,b a Department of Chemistry, North-Eastern Hill University, Shillong 793022, India B Centre for Computational Quantum Chemistry, University of Georgia, Athens GA 30602 ABSTRACT: This survey of metal-metal (MM) bond distances in binuclear complexes of the first row 3d-block elements reviews experimental and computational research on a wide range of such systems. The metals surveyed are titanium, vanadium, chromium, manganese, iron, cobalt, nickel, copper, and zinc, representing the only comprehensive presentation of such results to date. Factors impacting MM bond lengths that are discussed here include (a) n+ the formal MM bond order, (b) size of the metal ion present in the bimetallic core (M2) , (c) the metal oxidation state, (d) effects of ligand basicity, coordination mode and number, and (e) steric effects of bulky ligands. Correlations between experimental and computational findings are examined wherever possible, often yielding good agreement for MM bond lengths. The formal bond order provides a key basis for assessing experimental and computationally derived MM bond lengths. The effects of change in the metal upon MM bond length ranges in binuclear complexes suggest trends for single, double, triple, and quadruple MM bonds which are related to the available information on metal atomic radii. It emerges that while specific factors for a limited range of complexes are found to have their expected impact in many cases, the assessment of the net effect of these factors is challenging. -

Electron Ionization
Chapter 6 Chapter 6 Electron Ionization I. Introduction ......................................................................................................317 II. Ionization Process............................................................................................317 III. Strategy for Data Interpretation......................................................................321 1. Assumptions 2. The Ionization Process IV. Types of Fragmentation Pathways.................................................................328 1. Sigma-Bond Cleavage 2. Homolytic or Radical-Site-Driven Cleavage 3. Heterolytic or Charge-Site-Driven Cleavage 4. Rearrangements A. Hydrogen-Shift Rearrangements B. Hydride-Shift Rearrangements V. Representative Fragmentations (Spectra) of Classes of Compounds.......... 344 1. Hydrocarbons A. Saturated Hydrocarbons 1) Straight-Chain Hydrocarbons 2) Branched Hydrocarbons 3) Cyclic Hydrocarbons B. Unsaturated C. Aromatic 2. Alkyl Halides 3. Oxygen-Containing Compounds A. Aliphatic Alcohols B. Aliphatic Ethers C. Aromatic Alcohols D. Cyclic Ethers E. Ketones and Aldehydes F. Aliphatic Acids and Esters G. Aromatic Acids and Esters 4. Nitrogen-Containing Compounds A. Aliphatic Amines B. Aromatic Compounds Containing Atoms of Nitrogen C. Heterocyclic Nitrogen-Containing Compounds D. Nitro Compounds E. Concluding Remarks on the Mass Spectra of Nitrogen-Containing Compounds 5. Multiple Heteroatoms or Heteroatoms and a Double Bond 6. Trimethylsilyl Derivative 7. Determining the Location of Double Bonds VI. Library -
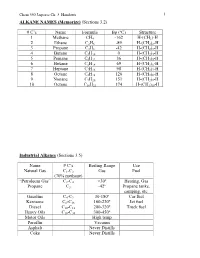
C's Name Formula Bp (ºC) Structure 1 Methane CH4 -162 H-(CH2)
Chem 350 Jasperse Ch. 3 Handouts 1 ALKANE NAMES (Memorize) (Sections 3.2) # C’s Name Formula Bp (ºC) Structure 1 Methane CH4 -162 H-(CH2)-H 2 Ethane C2H6 -89 H-(CH2)2-H 3 Propane C3H8 -42 H-(CH2)3-H 4 Butane C4H10 0 H-(CH2)4-H 5 Pentane C5H12 36 H-(CH2)5-H 6 Hexane C6H14 69 H-(CH2)6-H 7 Heptane C7H16 98 H-(CH2)7-H 8 Octane C8H18 126 H-(CH2)8-H 9 Nonane C9H20 151 H-(CH2)9-H 10 Octane C10H22 174 H-(CH2)10-H Industrial Alkanes (Sections 3.5) Name # C’s Boiling Range Use Natural Gas C1-C3 Gas Fuel (70% methane) “Petroleum Gas” C2-C4 <30º Heating, Gas Propane C3 -42º Propane tanks, camping, etc. Gasoline C4-C9 30-180º Car fuel Kerosene C8-C16 160-230º Jet fuel Diesel C10-C18 200-320º Truck fuel Heavy Oils C16-C30 300-450º Motor Oils High temp Paraffin Vacuum Asphalt Never Distills Coke Never Distills Chem 350 Jasperse Ch. 3 Handouts 2 Nomenclature of Alkanes (Sections 3.3) Systematic IUPAC Rules for Branched and Substituted Alkanes (Section 3.3B) 1. Longest continuous C-chain “core name” 2. Number core chain from an end nearest a substituent 3. Name substituents as “alkyl” groups: 4. Specify the location of substituents using numbers (hyphenate the #’s) • If >2 substituents, list alphabetically • Use di-, tri-, tetra- if the same substituent is repeated. (But ignore these in alphabetizing). Punctuation Notes: • Hyphenate numbers • Do not put a space between substituents and the core name Special Names for Some 3 or 4-carbon Substituents H3C CH3 CH Memorize H3C C H3C CH3 Isopropyl t-butyl or tert-butyl H2 H2 CH3 H3C C H3C C CH H3C CH Others C C C C CH3 H3C C H2 H H H2 2 2 H2 n-propyl n-butyl isobutyl s-butyl (n for "normal") Another Classification System Primary (1º): with one attached carbon Secondary (2º): with two attached carbons Tertiary (3º): with three attached carbons H C C C C C C C C H 1º H 2º C 3º Very Complex Substituents (Not responsible) Substituent: (1-ethyl-2,3-dimethylpentyl) Overall: 9-(1-ethyl-2,3-dimethylpentyl)nonadecane Chem 350 Jasperse Ch. -

Influence of Conjugation Axis on the Optical and Electronic Properties Of
Article pubs.acs.org/joc Influence of Conjugation Axis on the Optical and Electronic Properties of Aryl-Substituted Benzobisoxazoles † ‡ ‡ † † Brian C. Tlach, Aimeé L. Tomlinson, Alden G. Ryno, Dawn D. Knoble, Dana L. Drochner, † Kyle J. Krager, and Malika Jeffries-EL*, † Department of Chemistry, Iowa State University, Ames, Iowa 50010, United States ‡ Department of Chemistry, University of North Georgia, Dahlonega, Georgia 30597, United States *S Supporting Information ABSTRACT: Six different 2,6-diethyl-4,8-diarylbenzo[1,2-d:4,5-d′]bis(oxazoles) and four different 2,4,6,8-tetraarylbenzobisoxazoles were synthesized in two steps: a Lewis acid catalyzed orthoester cyclization followed by a Suzuki or Stille cross-coupling with various arenes. The influence of aryl group substitution and/or conjugation axis variation on the optical and electronic properties of these benzobis(oxazole) (BBO) compounds was evaluated. Structural modifications could be used to alter the HOMO, LUMO, and band gap over a range of 1.0, 0.5, and 0.5 eV, respectively. However, depending on the location and identity of the substituent, the HOMO level can be altered without significantly impacting the LUMO level. This is supported by the calculated frontier molecular orbitals. Our results indicate that the FMOs and band gaps of benzobisoxazoles can be readily modified either jointly or individually. ■ INTRODUCTION Among the aforementioned examples, the benzo[1,2-d:4,5- d′]bis(oxazole) (BBO)-based cruciforms are particularly During the past four decades, interest in the development of π- interesting, since these molecules have two different con- conjugated materials has increased due to their potential use as jugation pathways: 2,6-conjugation through the oxazole rings replacements for inorganic materials in a variety of semi- and 4,8-conjugation through the central benzene ring (Scheme conducting applications including field effect transistors − − 1). -

Introduction to Alkenes and Alkynes in an Alkane, All Covalent Bonds
Introduction to Alkenes and Alkynes In an alkane, all covalent bonds between carbon were σ (σ bonds are defined as bonds where the electron density is symmetric about the internuclear axis) In an alkene, however, only three σ bonds are formed from the alkene carbon -the carbon thus adopts an sp2 hybridization Ethene (common name ethylene) has a molecular formula of CH2CH2 Each carbon is sp2 hybridized with a σ bond to two hydrogens and the other carbon Hybridized orbital allows stronger bonds due to more overlap H H C C H H Structure of Ethylene In addition to the σ framework of ethylene, each carbon has an atomic p orbital not used in hybridization The two p orbitals (each with one electron) overlap to form a π bond (p bonds are not symmetric about the internuclear axis) π bonds are not as strong as σ bonds (in ethylene, the σ bond is ~90 Kcal/mol and the π bond is ~66 Kcal/mol) Thus while σ bonds are stable and very few reactions occur with C-C bonds, π bonds are much more reactive and many reactions occur with C=C π bonds Nomenclature of Alkenes August Wilhelm Hofmann’s attempt for systematic hydrocarbon nomenclature (1866) Attempted to use a systematic name by naming all possible structures with 4 carbons Quartane a alkane C4H10 Quartyl C4H9 Quartene e alkene C4H8 Quartenyl C4H7 Quartine i alkine → alkyne C4H6 Quartinyl C4H5 Quartone o C4H4 Quartonyl C4H3 Quartune u C4H2 Quartunyl C4H1 Wanted to use Quart from the Latin for 4 – this method was not embraced and BUT has remained Used English order of vowels, however, to name the groups -

(12) United States Patent (10) Patent No.: US 8,779,144 B2 Kelly Et Al
US00877914.4B2 (12) United States Patent (10) Patent No.: US 8,779,144 B2 Kelly et al. (45) Date of Patent: *Jul. 15, 2014 (54) BICYCLOHETEROARYL COMPOUNDSAS (56) References Cited P2X7 MODULATORS AND USES THEREOF U.S. PATENT DOCUMENTS (75) Inventors: Michael G. Kelly, Thousand Oaks, CA (US); John Kincaid, San Mateo, CA 5,095,103 A 3, 1992 Hoechstetter (US); Yunfeng Fang, Foster City, CA 5,484.926 A 1/1996 Dressman et al. 5,670.517 A 9/1997 Choy et al. (US); Jianhua He, legal representative, 6,110,934. A * 8/2000 Harling et al. ................ 514,307 Foster City, CA (US); Yeyu Cao, Foster 7,402,596 B2 7/2008 Kelly et al. City, CA (US); Carl Kaub, San Mateo, CA (US); Sumithra Gowlugari, San FOREIGN PATENT DOCUMENTS Mateo, CA (US) DE 39 42 893 A1 6, 1991 (73) Assignee: Evotec (US) Inc., South San Francisco, JP 2006220522 8, 2006 CA (US) WO WO94/17066 8, 1994 WO WO94,27969 12/1994 (*) Notice: Subject to any disclaimer, the term of this WO WO95/09843 4f1995 patent is extended or adjusted under 35 WO WOO3,068743 8, 2003 U.S.C. 154(b) by 1644 days. WO WOO3,080579 10, 2003 WO WO 2004/106305 12, 2004 This patent is Subject to a terminal dis WO 2005.009968 2, 2005 claimer. WO WO 2005/087767 9, 2005 WO WO 2005/087768 9, 2005 Appl. No.: 11/722,514 WO WO 2005,105814 11, 2005 (21) WO WO 2005.115986 12/2005 WO WO 2006/005.941 1, 2006 (22) PCT Fled: Mar.